Antibody or antibody fragment specifically binding to voltage-gated sodium channel alpha subunit Nav1.7
A sodium ion channel and antibody fragment technology, which is applied in the field of antibodies and/or antibody fragments that specifically recognize the above-mentioned targets, can solve problems such as research difficulties, side effects that are difficult to overcome, and cardiotoxicity, and achieve overcoming side effects and good targeting , therapeutic and pain-relieving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Antigen Synthesis
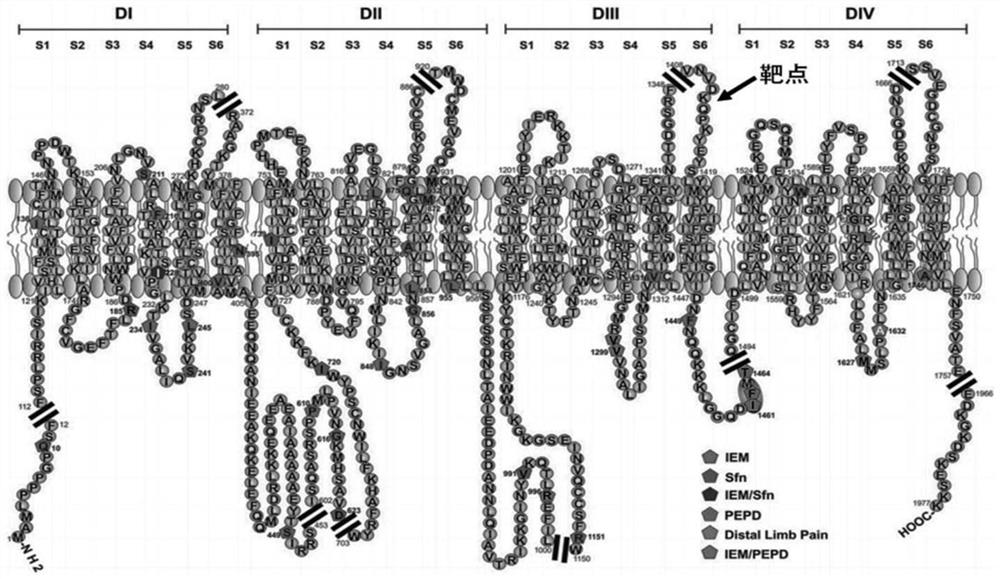
[0048] According to the amino acid sequence of Nav 1.7 (GenBank No.NP_002968) and the functional region of the crystal structure ( figure 1 ) was analyzed for hydrophilicity and antigenicity, and the DSVNVDKQPKYEYS sequence was screened out, and its hydrophilicity and antigenicity met the requirements of the antigen, and the CDSVNVDKQPKYEYS (SEQ ID NO.9) polypeptide was artificially synthesized by using an automatic synthesizer.
[0049] Specific steps are as follows:
[0050] (1) Connect -COOH of the first AA to Cl-Resin with DIEA, and then block the unreacted functional groups on the resin with MeOH;
[0051] (2) washing with DMF;
[0052] (3) Use Pip to remove -NH in the first AA 2 The protecting group Fmoc makes -NH 2 exposed;
[0053] (4) washing with DMF;
[0054] (5) Activate -COOH of the second AA with DIC+HOBT, and then condense to -NH in the first AA 2 on, forming an amide bond;
[0055] (6) washing with DMF;
[0056] (7...
Embodiment 2
[0063] Embodiment 2 monoclonal cell line preparation
[0064] 2.1 Animal immunity
[0065] Prepare Freund's complete adjuvant Sigma, F5881 and Freund's incomplete adjuvant (Sigma, F5506). Using the end-SH of polypeptide C9797BL020-7, the polypeptide was coupled to the carrier protein KLH as an immunogen.
[0066] Five 8-week-old female BALB / c (animal numbers: #4061, #4062, #4063, #4064, #4065) were selected for three times of intraperitoneal immunization to stimulate the body to produce an immune response to produce antibodies. Primary immunization: 50 μg / monkey, followed by secondary immunization three weeks later, with a dose of 50 μg / bird; 2 weeks after the second immunization, the third immunization was performed, with a dose of 50 μg / bird, within 1 day of the third immunization. A week later, blood was drawn for antibody testing.
[0067] 2.2 Animal serum ELISA detection
[0068] 2.2.1 Instruments and equipment:
[0069] Plate washer: Beijing Nanhua ZDMX
[0070] Mi...
Embodiment 3
[0103] Example 3 Detection of antibody immunogenicity
[0104] 3.1 Antigen preparation
[0105] 3.1.1: Vector construction and crude protein preparation
[0106] Synthesize 80 amino acid peptides containing the ion conduction pore module target of the DIVS3 domain of Nav1.7, construct the prokaryotic expressed His fusion protein expression vector, inoculate in 2000mL LB liquid medium (Kana resistance), at 37 degrees Shake culture overnight, when OD600 is about 0.6, lower the culture temperature to 30°C; add IPTG inducer to a final concentration of 0.1mM, continue shaking culture at 30°C for 8h; collect bacteria by centrifugation for 3min, resuspend in 50mL pre-cooled NTA-0 buffer solution, after 30 minutes in ice bath, sonicate the bacterial cells, centrifuge at 4°C for 50 minutes, and collect the precipitate (inclusion body); , centrifuge at 4°C for 10 min, remove the supernatant, and obtain the crude protein preparation;
[0107] 3.1.2 Protein denaturation and renaturatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com