Preparation method of high-purity sugammadex sodium
A sugammadex sodium, high-purity technology, which is applied in the chemical and pharmaceutical field, can solve problems such as complex by-products and degradation impurities, difficult purification of sugammadex sodium, and high-purity products, so as to improve the total purity and process Easy handling, no effects of highly toxic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
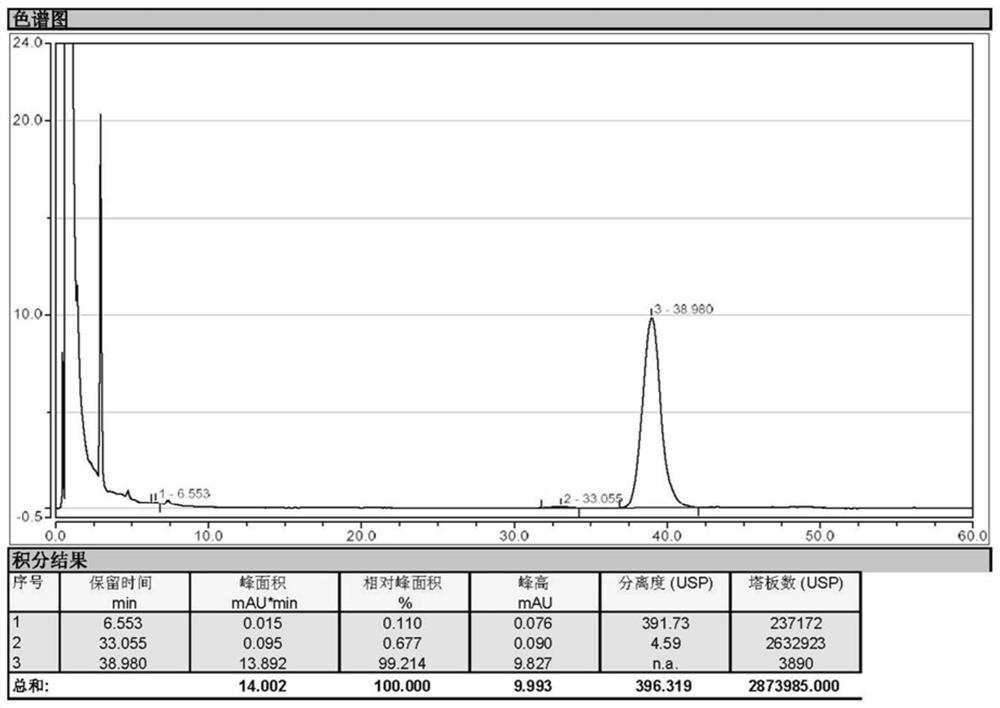
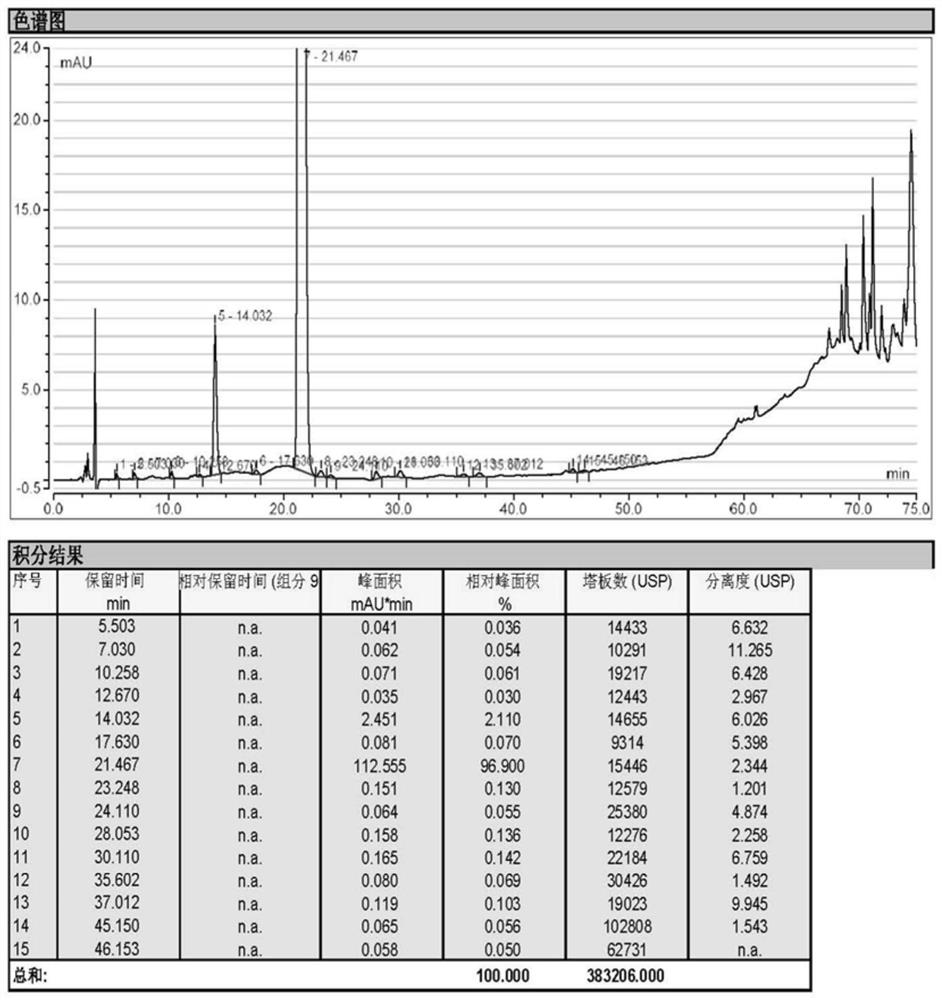
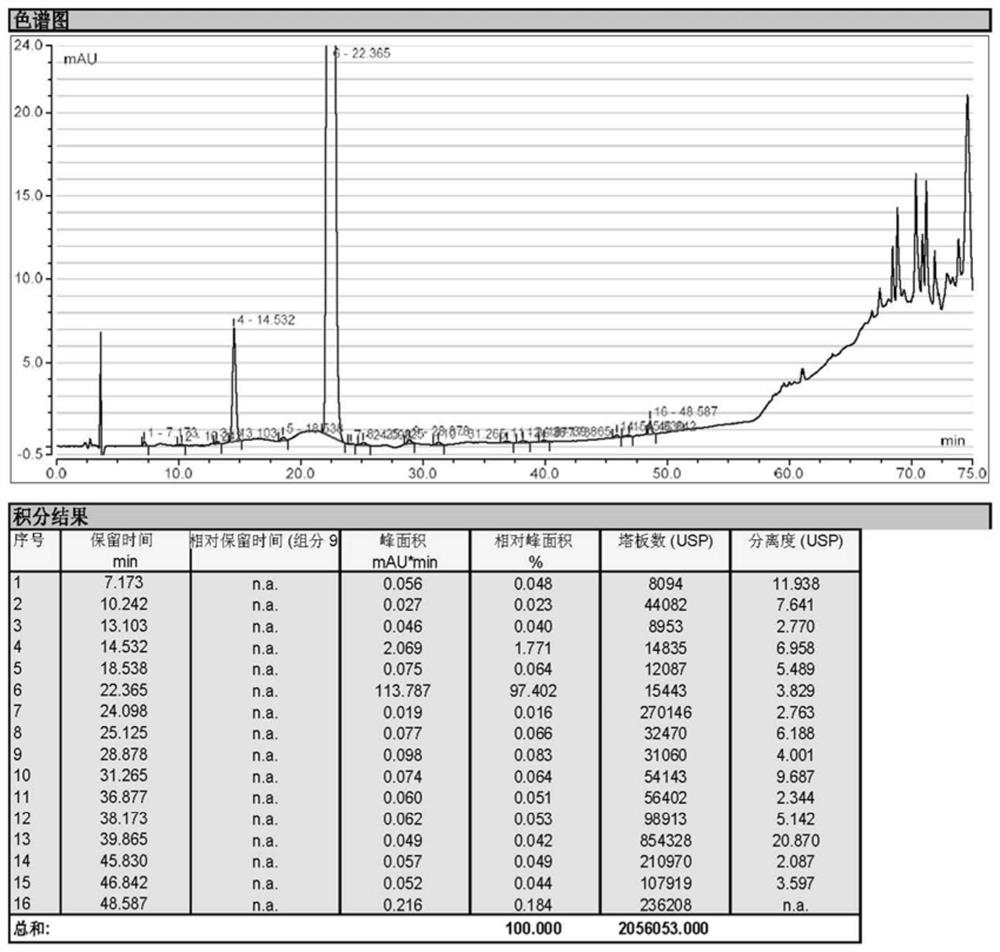
Image
Examples
Embodiment 1
[0060] Embodiment 1: the preparation of Vilsmeier Haack reagent
[0061] In the four-neck flask, nitrogen protection was passed through, and 756.8g of triphenylphosphine and 2356ml of dry DMF were successively added at room temperature (20-25°C), and stirred to dissolve. Under nitrogen protection at room temperature (20-25°C) and stirring, 465.85g of bromine was slowly added dropwise, and the temperature was kept below 40°C during the dropwise addition. After the dropwise addition, stir and react at room temperature (20-25°C) for 1 hour, then cool down to 0°C, and under nitrogen protection, filter the reactants in the four-necked bottle, and rinse the filter cake with 0°C DMF 1200ml×2 Twice, then rinse the filter cake twice with 1200ml x 2 of dry methyl tert-butyl ether, and vacuum-dry at room temperature for 5h to obtain 440g of dry Vilsmeier Haack reagent for use.
[0062]
Embodiment 2
[0063] Example 2: Preparation of 6-perdeoxy-6-perbromo-γ-cyclodextrin
[0064] In a four-neck flask, under nitrogen protection, add 100 g of dry γ-cyclodextrin and 1200 ml of DMF in sequence at room temperature, and stir to dissolve. Cool down to 0°C, under nitrogen protection and stirring, add the Vilsmeier Haack reagent of Example 1 in five batches, add 73g each time for the first four times, stir and react for 5min after adding, then add the next time, add 76.73g for the fifth time, and always Keep the temperature when adding the Vilsmeier Haack reagent should not exceed 25°C. After the addition was completed and stirred for 5 min, the temperature of the system was raised to 73° C., and stirred for 6 h under the protection of nitrogen. After the reaction was completed, the temperature was lowered to 40° C., and 140 ml of water was slowly added dropwise to quench the reaction (30 min), and stirring was continued at this temperature for 3.5 h. 1200ml of water was added drop...
Embodiment 3
[0067] Embodiment 3: preparation sugammadex sodium crude product
[0068] In a 500ml four-neck bottle, pass through nitrogen protection, add 50g of perbrominated intermediate at room temperature, then add 1000ml of dimethyl sulfoxide, and stir until it dissolves. 29.5 g of 3-mercaptopropionic acid were added with stirring. Add 166.7ml of 20% sodium hydroxide aqueous solution dropwise to the reaction solution under stirring (the dropping time is not less than 15 minutes, and the dropping temperature is lower than 30°C), and after stirring at room temperature for 5 minutes, the reaction system is heated to 50°C, and the temperature is kept for reaction 6 hours. After the reaction, the system was cooled to room temperature, and 2500 ml of ethanol was added dropwise with stirring. Solids were precipitated, and the stirring was continued for 10 minutes, filtered, and the filter cake was washed twice with a small amount of ethanol. At room temperature, transfer the solid obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com