Electrode-forming material for electrochemical capacitors
A capacitor and electrochemical technology, applied in the field of electrode formation materials for electrochemical capacitors, can solve the problems of rare ruthenium oxide, low battery voltage, high cost, etc., and achieve the effects of preventing power generation loss, large storage capacity, and long cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
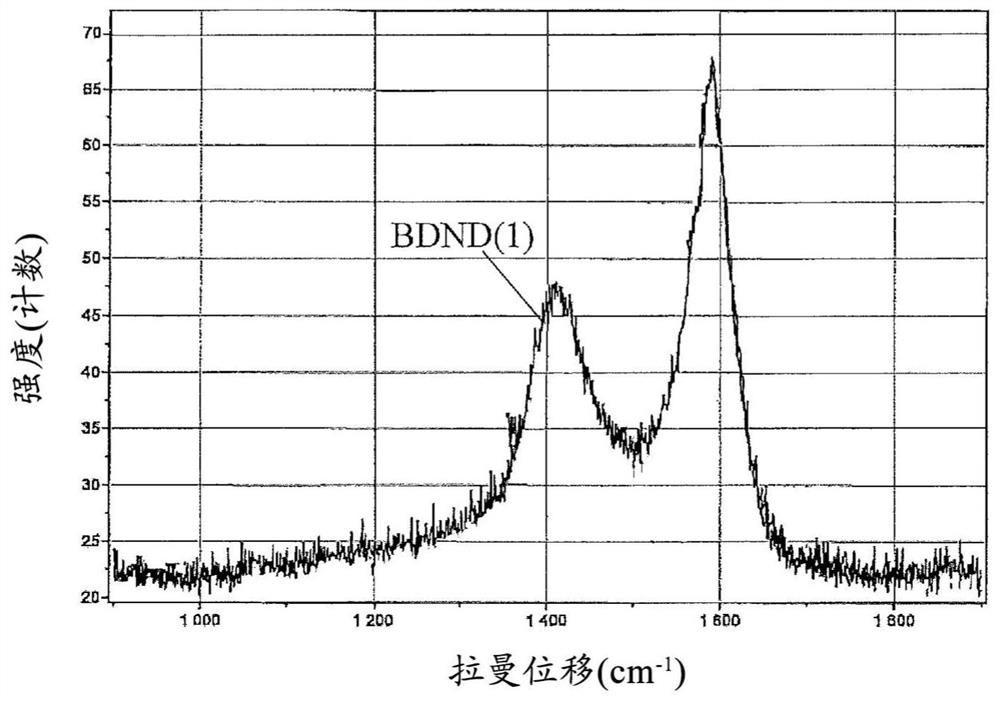
[0128] Preparation Example 1 (Manufacture of BDND)
[0129] (generation process)
[0130] First, an electric detonator is attached to the formed explosive and placed in a pressure-resistant container for detonation (iron container, volume: 15 m 3 ), seal the container tightly. As the explosive, 0.50 kg of a mixture of TNT and RDX (TNT / RDX (mass ratio)=50 / 50) was used. Next, the electric detonator is detonated to detonate the explosive in the container. Next, let stand at room temperature for 24 hours to cool the container and its interior. After this natural cooling, the operation of scraping off the ND crude product (including the aggregates of ND particles and soot) adhering to the inner wall of the container with a scraper was performed, and the ND crude product was recovered. The recovered amount of ND crude product was 0.025 kg.
[0131] (Oxidation treatment step)
[0132] Next, the ND crude product ( 3g), concentrated sulfuric acid (80.6g) and copper carbonate (ca...
preparation example 2
[0156] Preparation Example 2 (Production of Ruthenium Oxide Nanoparticles)
[0157] RuCl in 0.1M at 0.5mL / min 3 To 100 mL, 30 mL of a 1 M aqueous NaOH solution was added. It was then stirred for 12 hours.
[0158] Then, the reaction liquid was subjected to centrifugation treatment (7500 rpm×15 minutes) to remove water, and the filtrate was further removed by suction filtration to obtain a filtrate.
[0159] The obtained filtrate was washed with water and dried under reduced pressure (at 0.08 MPa and 120° C. for 12 hours or more) to obtain ruthenium oxide nanoparticles (1). The particle diameter (D50) of the obtained ruthenium oxide nanoparticles (1) was measured by the dynamic light scattering method. The result is 50nm.
Embodiment 1
[0160] Example 1 (Production of Electrode Forming Material)
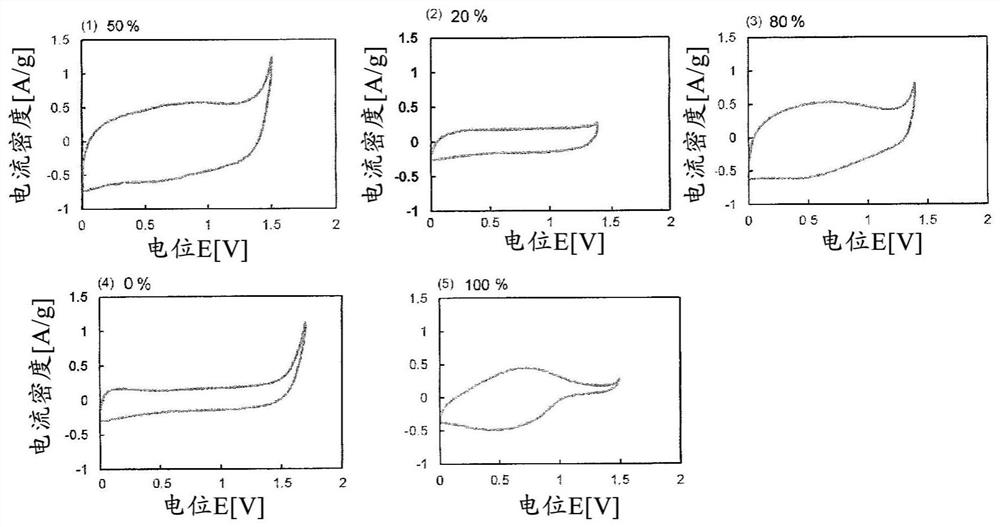
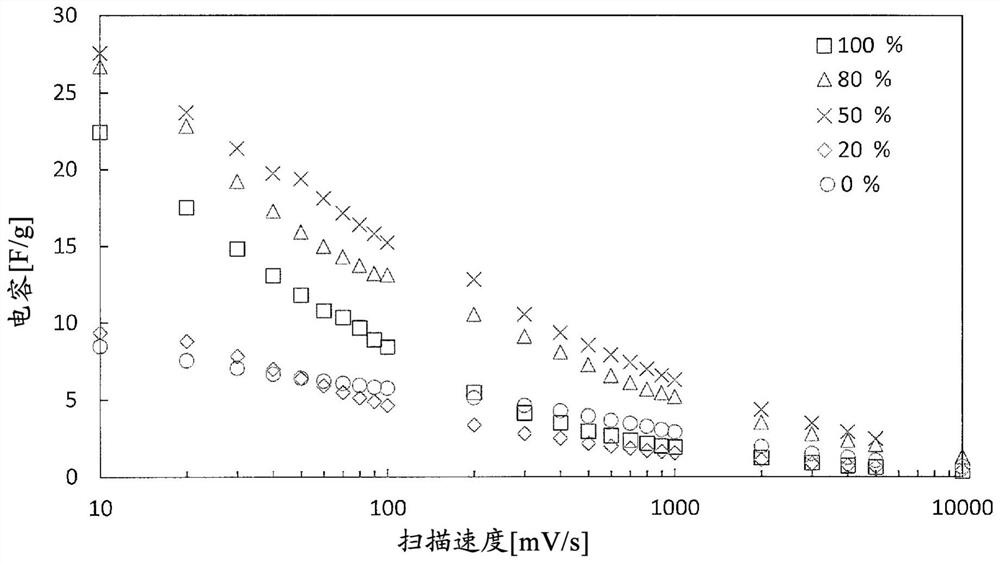
[0161] 5 mg of the obtained BDND (1) and 5 mg of the ruthenium oxide nanoparticles (1) were dispersed in 0.5 mL of 30% by mass ethanol to obtain an electrode-forming material (1) (B / (A+B): 50%).
[0162] 20 μL of the obtained electrode-forming material (1) was cast on a glassy carbon substrate as a current collector, and after heating and drying at 60° C., 5 mass % Nafion (having a hydrophobic Teflon (registered trademark) 10 μL of perfluorocarbon having a perfluoro side chain having a sulfonic acid group bonded to the skeleton was poured onto the outermost surface to obtain an electrode (1) (per 1 cm 2 BDND loading of the electrode: 2.9 mg, 2.9 mg of ruthenium oxide nanoparticles).
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com