Antibacterial peptide P104 and lyase LysP53 with broad-spectrum splitting activity, and application of antibacterial peptide P104 and lyase LysP53
A technology of P104 and lyase, which is applied in the direction of lyase, medical preparations containing active ingredients, applications, etc., can solve the problems of less research on phage lyase, and achieve high enzyme activity, good cleavage effect, and good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The synthesis of embodiment 1 antimicrobial peptide P104
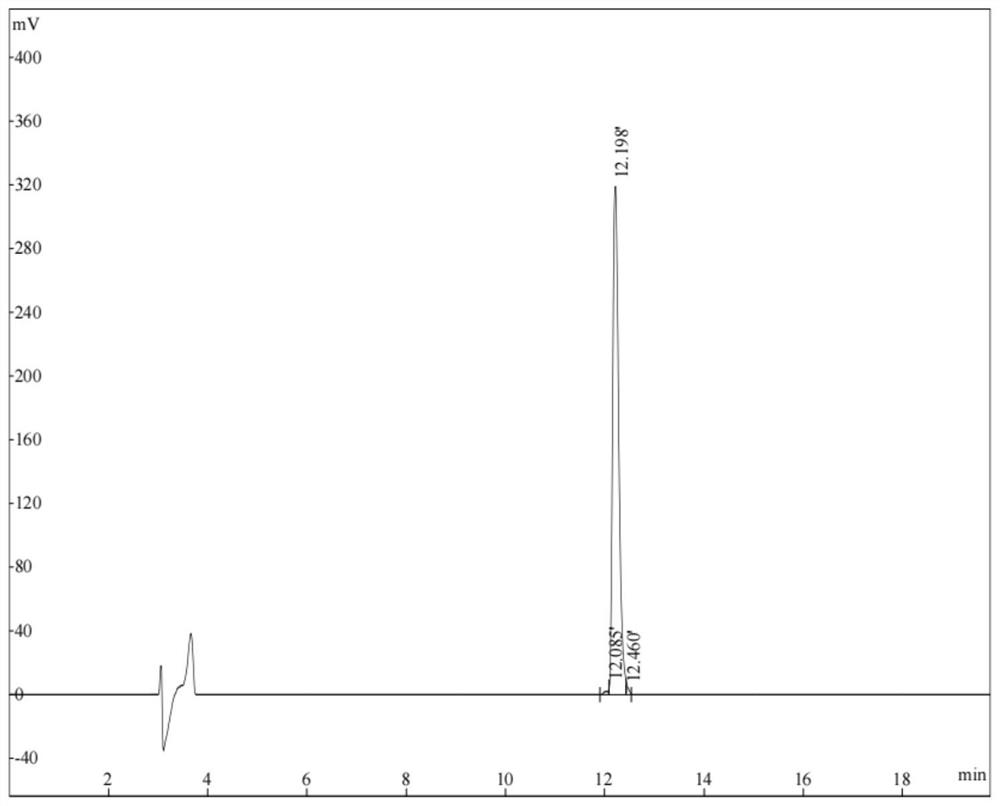
[0043] After a large number of experimental analysis by the inventor, the inventor found a section of antimicrobial peptide P104 from the phage P53 genome, the amino acid sequence of the antibacterial peptide P104 is shown in SEQ ID NO.1; the gene sequence of the antibacterial peptide P104 is shown in SEQ ID NO. 3. The antimicrobial peptide P104 was synthesized by Shanghai Qiangyao Biotechnology Co., Ltd. The purity of the synthesized antimicrobial peptide P104 was 98.58%, and the molecular weight was 3885.57. The purity of the antimicrobial peptide P104 was determined by high performance liquid chromatography. The chromatographic purity of the antimicrobial peptide P104 picture see figure 1, and the analysis results are shown in Table 1 below,
[0044] Table 1 Antimicrobial Peptide P104 Liquid Chromatography Detection Component List
[0045] retention time (min) Peak area purity(%) 12.085 ...
Embodiment 2
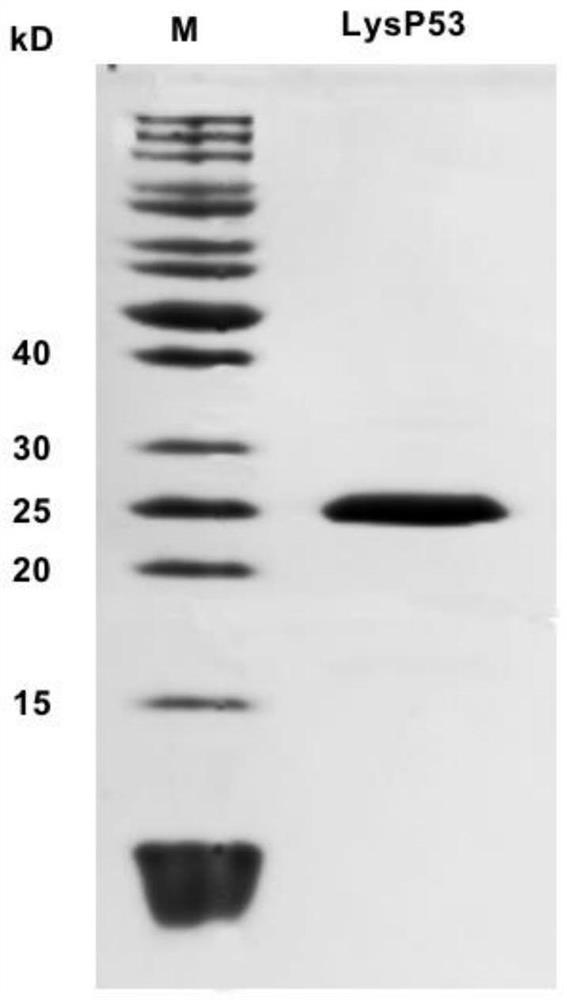
[0050] Example 2 Expression and purification of lyase LysP53
[0051] After a large number of experimental analysis by the inventor, the inventor found a lyase LysP53 from the phage P53 genome, the amino acid sequence of the lyase LysP53 is shown in SEQ ID NO.2; the gene sequence of the lyase LysP53 is shown in SEQ ID NO .4 shown.
[0052] 2.1 Construction of recombinant expression vector
[0053] According to the gene sequence of the lyase LysP53, the conventional primer design software in the prior art was adopted to design the following primer sequences:
[0054] 53P37-F: ctttaagaaggagatataccatggATGACGATGACAACAAAACGTA (as shown in SEQ ID NO.5, has an NcoI restriction site);
[0055] 53P37-R: tggtggtggtggtggtgctcgagCCCCGCCAATTCAAAGTGTGGGCT (as shown in SEQ ID NO. 6, with an XhoI restriction site).
[0056] The target gene fragment can be synthesized by a biological company, or the phage P53 genome can be used as a template to amplify the target gene. In the present invent...
Embodiment 3
[0060] Example 3 The influence of time on the activity of lyase LysP53 to crack A.baumannii WHG40137
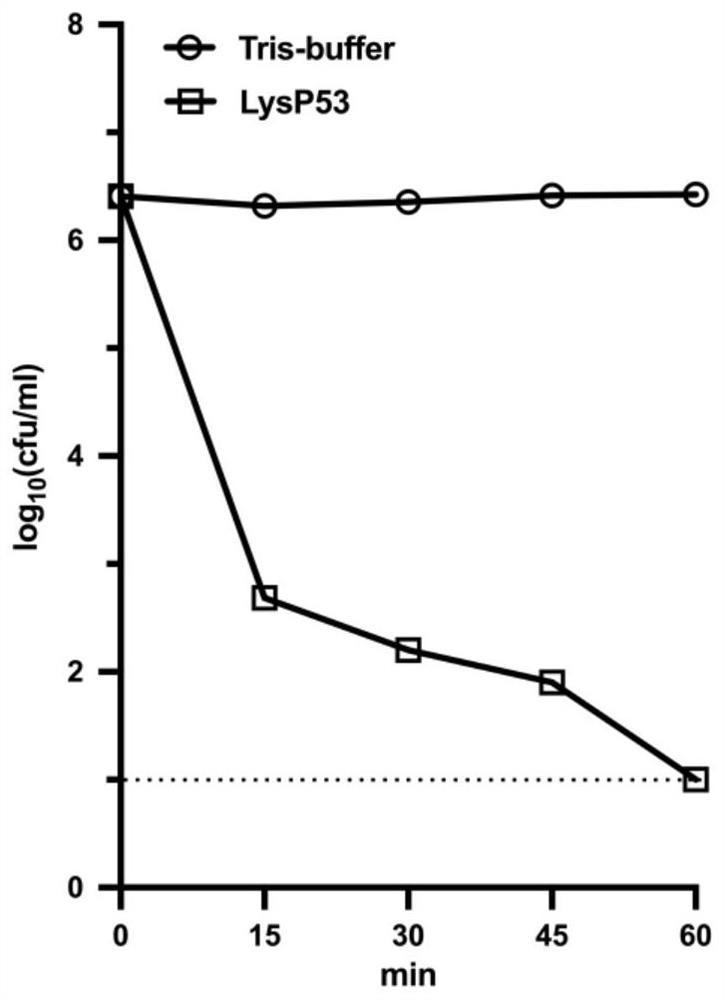
[0061] Acinetobacter baumannii A.baumannii WHG40137 was cultivated to the logarithmic phase (OD 600nm =0.4~0.6), after the low-temperature centrifugation collects the precipitate, after washing once with Tris-HCl buffer solution, then the precipitate after washing is dissolved in the above-mentioned buffer solution to obtain the A.baumannii WHG40137 bacterial liquid, which is obtained in Example 2 The lysing enzyme LysP53 was mixed with the above-mentioned bacterial solution so that the final concentration of the lysing enzyme LysP53 was 100 μg / ml. At the same time, the mixture of the same amount of buffer and the above-mentioned bacterial solution was used as a negative control, and incubated at 37°C. Sampling at time points, and counting plate counts, the results obtained are shown in image 3 .
[0062] From image 3 It can be seen from the results that after incubation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com