Hybridoma cell strain capable of stably secreting monoclonal antibody against novel coronavirus nucleocapsid protein as well as establishment method and application of hybridoma cell strain
A hybridoma cell line and monoclonal antibody technology, applied in the field of hybridoma cell line and its establishment, can solve the problems of loss, decreased ability to secrete monoclonal antibody, monoclonal antibody single reactivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
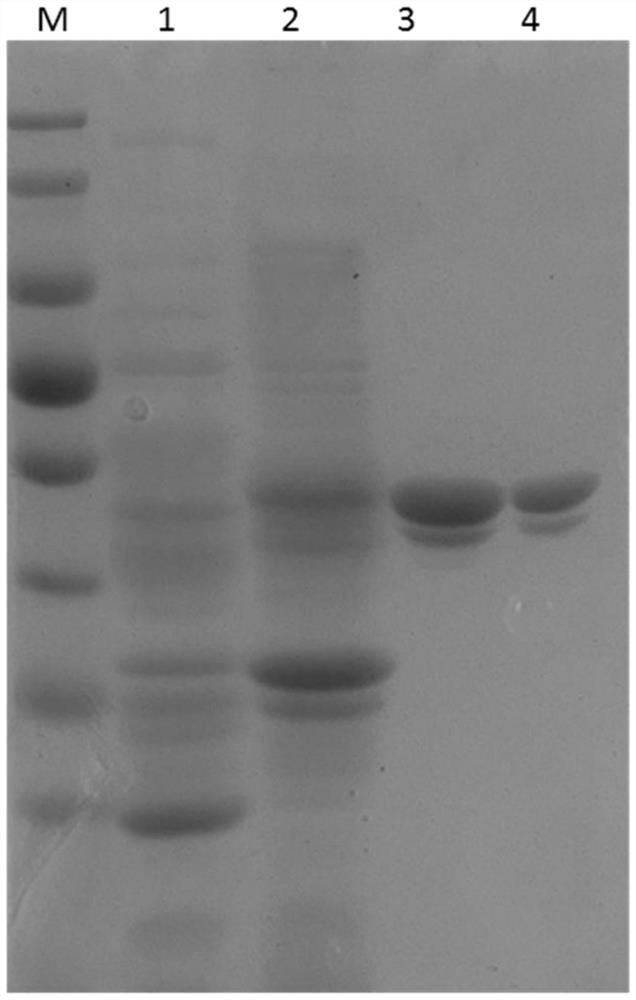
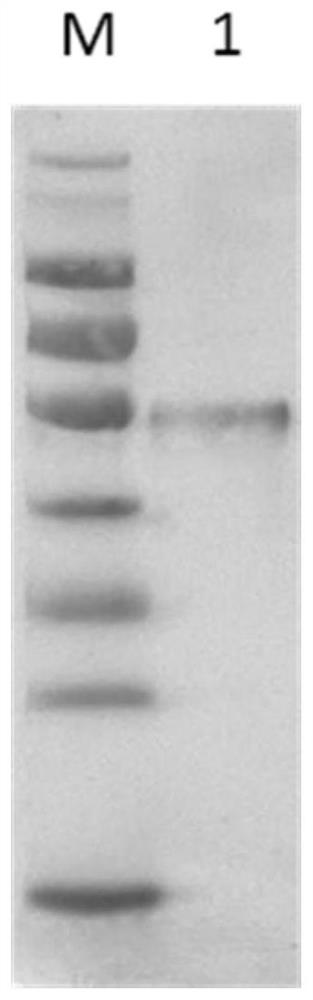
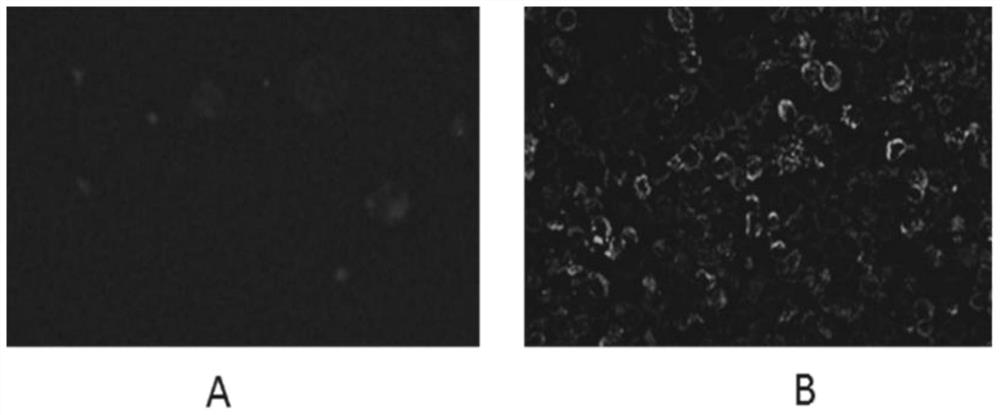
Image
Examples
Embodiment 1
[0047] Establishment of hybridoma cell lines for anti-new coronavirus nucleocapsid protein monoclonal antibody:
[0048] 1. Recombinant nucleoprotein
[0049] According to the published SARS-CoV-2 genome sequence, a gene sequence encoding the SARS-CoV-2 nucleocapsid protein suitable for expression in Escherichia coli was artificially synthesized and cloned into the expression vector pET28a. Transform competent Escherichia coli BL-21 with the recombinant expression vector, apply LB kanamycin plate, and culture overnight at 37°C, pick a single colony the next day, inoculate in 3ml LB culture medium, and culture at 37°C Shake culture for 16 hours, extract the plasmid, and carry out gene sequencing. Transform the recombinant expression plasmid with correct sequencing into BL-21 bacteria, inoculate it into LB medium tubes containing 50 μg / ml kanamycin, culture it with shaking at 37°C overnight, and then inoculate 1% of the inoculum into the tubes containing 50μg / ml Kanamycin 200m...
Embodiment 2
[0071] ELISA kit for detection of new crown IgM antibody using nCoVN3G6 monoclonal antibody
[0072] Dilute goat anti-human IgM antibody with 1:500 phosphate buffer solution (PBS pH7.2), add 100 μL per well to a 96-well ELISA reaction plate, coat at 4°C overnight, then add 100 μL 3% BSA to each well to block for 1 hour at room temperature , wash the ELISA plate 3 times with PBS-Tween20, add 100 μL of 1:100 diluted patient serum, react at 37°C for 1 hour, wash the ELISA plate 3 times with PBS-Tween20, add 100 μL (25ng) of recombinant SARS-CoV-2--N protein, 37 React for 1 hour at ℃, wash the ELISA plate 3 times with PBS-Tween20, dilute horseradish peroxidase (HRP)-labeled nCoVN3G6 monoclonal antibody, react for 1 hour at 37℃, wash the ELISA plate 3 times with PBS-Tween20, add HRP base (ABTS or TMB or OPD), stop the reaction after 30 minutes of chromogenic reaction at 37°C, measure the absorbance OD value of each sample well with a microplate reader, if the OD value is more than ...
Embodiment 3
[0075] ELISA kit for detection of SARS-CoV-2 virus antigen by double antibody sandwich method using nCoVN3G6 monoclonal antibody
[0076] Add nCoVN3G6 monoclonal antibody diluted with 1:10000 phosphate buffered saline (PBS pH7.2), add 100 μL per well to a 96-well ELISA reaction plate, coat at 4°C overnight, then add 100 μL 3% BSA to each well to block at room temperature for 1 hour, Wash the ELISA plate 3 times with PBS-Tween20, add 100 μL patient’s nasopharyngeal swab sample preservation solution, react at 37°C for 1 hour, wash the ELISA plate 3 times with PBS-Tween20, add 100 μL diluted horseradish peroxidase (HRP)-labeled nCoVN3G6 monoclonal antibody, react at 37°C for 1 hour, wash the ELISA plate 3 times with PBS-Tween20, add HRP substrate (ABTS or TMB or OPD), stop the reaction after 30 minutes of color reaction at 37°C, and measure each sample with a microplate reader Well absorbance OD value, OD value more than 2 times higher than the negative control was judged as posi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com