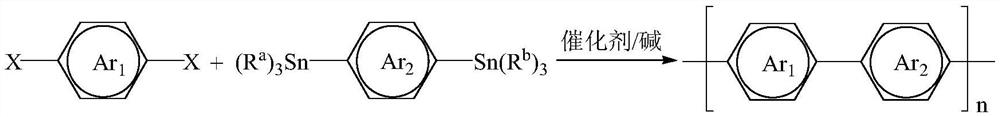Stille cross-coupling room-temperature polymerization method based on aryl dihalide and aryl distannane
An aryldihalide and aryldistanane technology, which is applied in the field of organic semiconductor active layer donor-acceptor conjugated polymer synthesis, can solve the problem of reduced charge mobility, increased energy consumption, and copolymerization of D-A copolymers. structural defects and other problems, to achieve the effect of reducing synthesis cost, safety assurance, and reducing structural defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
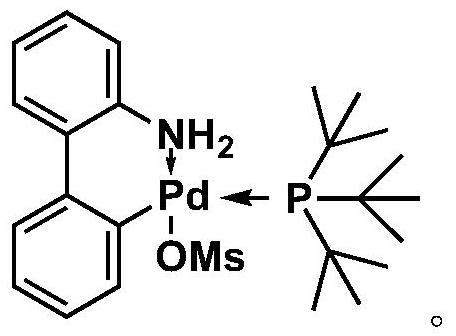
Method used
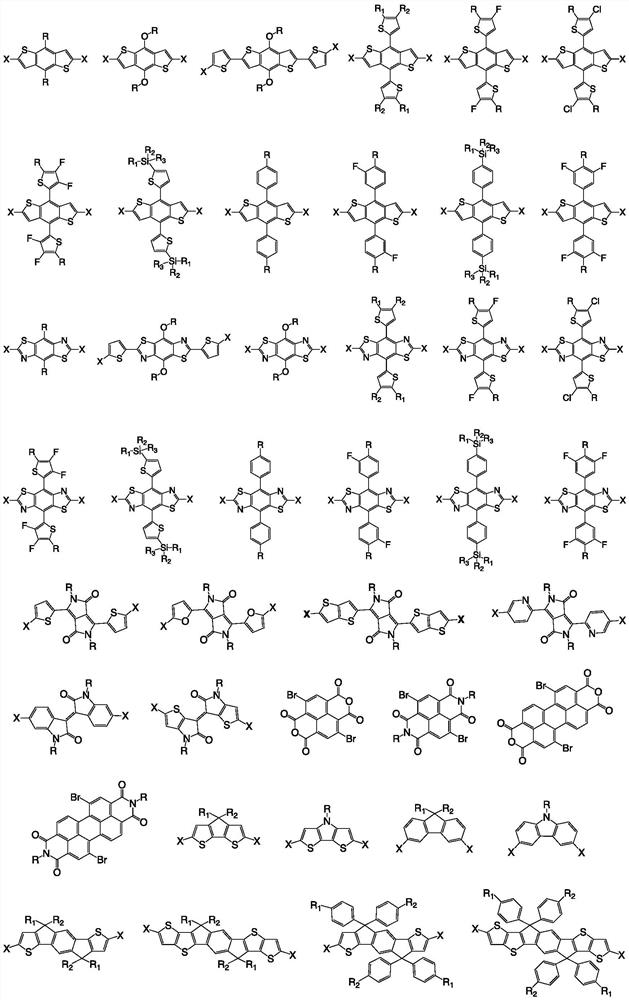
Image
Examples
Embodiment 1
[0032]
[0033] Under nitrogen protection, 2,5-bis(trimethyltin-based)selenophene (23.0 mg, 0.05 mmol), 3,6-bis(5-bromothienyl)-2,5-bis(2-decyl Tetradecyl)pyrrolo[3,4-c]pyrrole-1,4-dione (56.4mg, 0.05mmol), P(t-Bu) 3 Pd G3 (2.9 mg, 0.005 mmol) and potassium phosphate (10.6 mg, 0.05 mmol) were placed in a reaction vial. After adding 1.0 mL of tetrahydrofuran, the mixture was stirred at room temperature for 24 hours. After adding 20.0 mL of methanol, the resulting precipitate was filtered. Then the precipitated polymer was subjected to Soxhlet extraction with acetone, n-hexane and chloroform, respectively. The solution obtained after extraction with chloroform was concentrated, and then the polymer was precipitated with 20.0 mL of methanol, filtered and dried to obtain a black-green polymer solid (52.2 mg, 95%). 1 H NMR (500MHz, CDCl 3 ): δ9.27-8.86(br,2H),7.49-6.73(br,4H),4.62-3.47(br,4H),2.07-1.96(br,2H),1.85-0.98(br,80H),0.99 -0.68(br,12H). GPC: M n 39.4kDa, M w 8...
Embodiment 2
[0035]
[0036] Under nitrogen protection, 2,5-bis(trimethyltin-based)selenophene (23.0 mg, 0.05 mmol), 3,6-bis(5-bromothiophen-2-yl)-2,5-bis( 2-octyldodecyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (50.8mg, 0.05mmol), P(t-Bu) 3Pd G3 (2.9 mg, 0.005 mmol) and potassium phosphate (10.6 mg, 0.05 mmol) were placed in a reaction vial. After adding 1.0 mL of tetrahydrofuran, the mixture was stirred at room temperature for 24 hours. After adding 20.0 mL of methanol, the resulting precipitate was filtered. Then the precipitated polymer was subjected to Soxhlet extraction with acetone, n-hexane and chloroform, respectively. The solution obtained after extraction with chloroform was concentrated, and then the polymer was precipitated with 20.0 mL of methanol, filtered and dried to obtain a black-green polymer solid (46.5 mg, 94%). 1 H NMR (500MHz, CDCl 3 ):δ9.26-8.82(br,2H),7.58-6.72(br,4H),4.34-3.65(br,4H),2.09-1.80(br,2H),1.53-0.98(br,64H),0.93 -0.74(br,12H). GPC: M n 32....
Embodiment 3
[0038]
[0039] Under nitrogen protection, 2,5-bis(trimethyltin-based)selenophene (23.0mg, 0.05mmol), 3,6-bis(5-bromothiophene[3,2-b]thiophen-2-yl )-2,5-bis(2-octyldodecyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (56.4mg, 0.05mmol), P (t-Bu) 3 Pd G3 (2.9 mg, 0.005 mmol) and potassium phosphate (10.6 mg, 0.05 mmol) were placed in a reaction vial. After adding 1.0 mL of tetrahydrofuran, the mixture was stirred at room temperature for 24 hours. After adding 20.0 mL of methanol, the resulting precipitate was filtered. Then the precipitated polymer was subjected to Soxhlet extraction with acetone, n-hexane and chloroform, respectively. The solution obtained after extraction with chloroform was concentrated, and then the polymer was precipitated with 20.0 mL of methanol, filtered and dried to obtain a black-green polymer solid (51.5 mg, 89%). 1 H NMR (500MHz, CDCl 3 ):δ9.51-8.63(br,2H),7.51-5.82(br,4H),4.55-3.50(br,4H),2.49-2.11(br,2H),2.09-1.00(br,64H),1.00 -0.65(br,12H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com