Small molecule compound for inhibiting aggregation of amyloid beta protein as well as preparation method and application of small molecule compound
A technology of small molecule compounds and β proteins, applied in the field of biomedicine, can solve problems such as poor biocompatibility, large molecular weight, and self-aggregation of polypeptides, and achieve the effects of prolonging life, removing movement disorders, and inhibiting aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
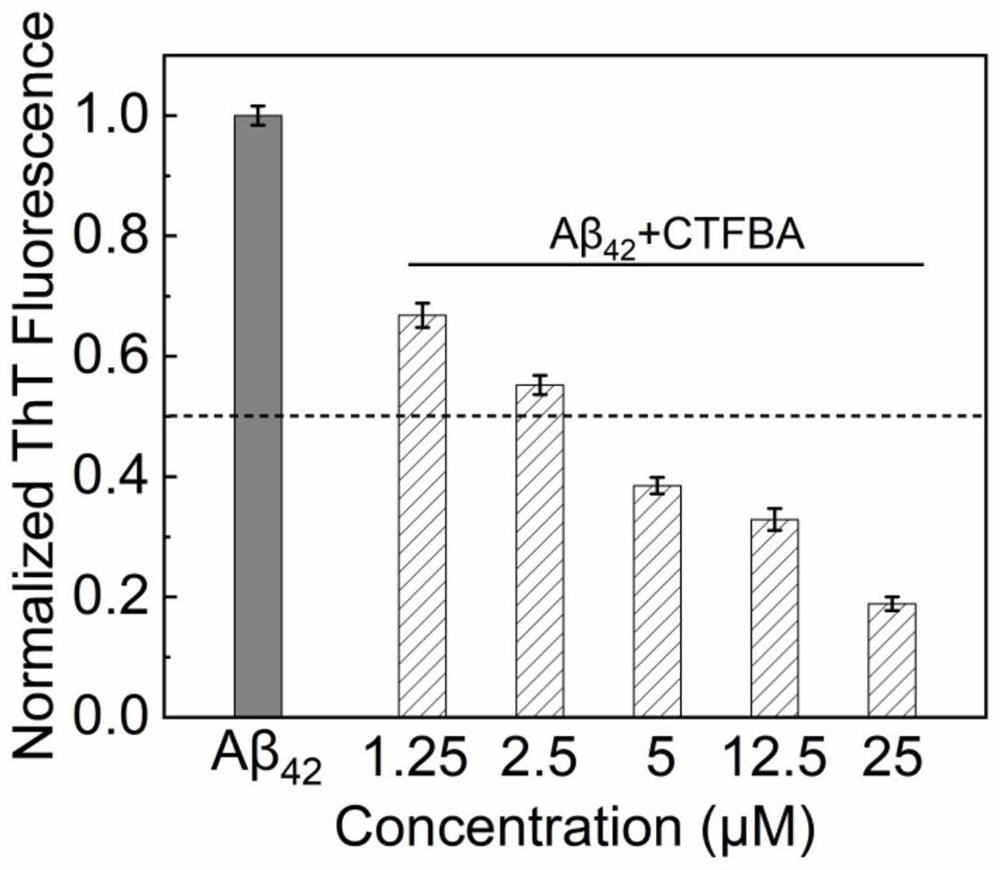
[0028] Example 1: Different concentrations of CTFBA and Aβ 42 ThT fluorescence intensity of the culture after co-cultivation for 48h.
[0029] Aβ with a purity of 95% 42 Dissolve in hexafluoroisopropanol (hexafiuro-2-isopropanol, HFIP) at a concentration of 1.0 mg / mL, destroy the formed aggregates by ultrasonication in an ice bath for 30 min, let stand at 4°C for 2 h to fully dissolve, and then Centrifuge at 4°C and 16,000 g for 20 minutes, take 75% of the supernatant and place it in a -70°C refrigerator overnight to freeze solid. Finally put into the freeze dryer, the Aβ 42 Freeze-dried into cotton flocs and stored in -20°C refrigerator.
[0030] freeze-dried Aβ 42 Dissolve in 20mM NaOH solution, sonicate in an ice bath for 10min to fully dissolve it, and obtain Aβ with a concentration of 275μM 42 mother liquor. Dilute with 27.5 μM ThT in HEPES buffer (20 mM, pH 7.4, containing 100 mM NaCl) to obtain a final concentration of 25 μM Aβ 42 solution as a control experiment...
Embodiment 2
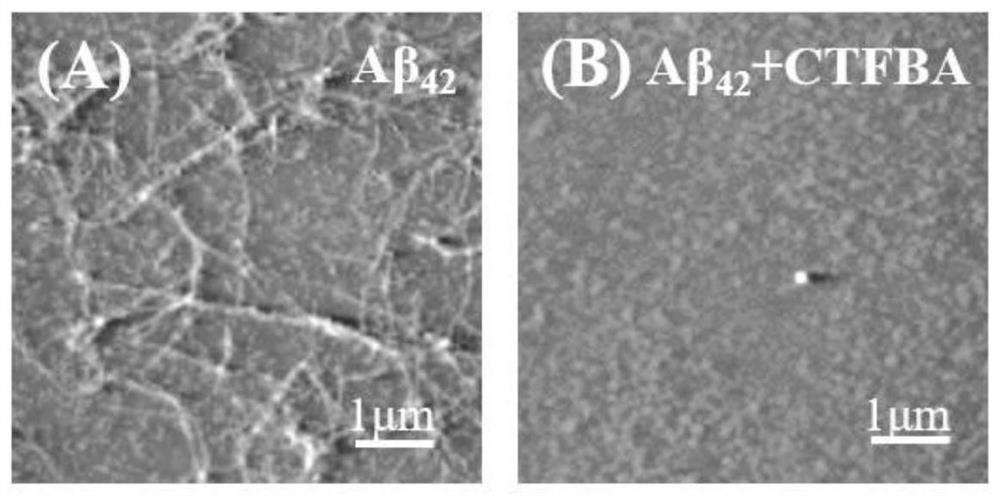
[0034] Example 2: CTFBA on Aβ 42 Aggregation effect.
[0035] Configure Aβ according to the method of Example 1 42 Stock solution, diluted with HEPES buffer (20 mM, pH 7.4, containing 100 mM NaCl) to obtain a final concentration of 25 μM Aβ 42 solution. Reconstitute Aβ containing 25 μM CTFBA 42 solution, Aβ in solution 42 The final concentration was 25 μM. The above solution was incubated at 37° C. and 150 rpm. After culturing for 48 hours, take 20 μL and drop it on the mica sheet, and let it stand for 10 minutes to fully combine the sample with the mica surface. Then wash slowly 7 times with deionized water to remove the salt ions in the buffer, and let it stand to dry. Observation was performed using the tapping mode of an atomic force microscope (CSPM5500, Yuanyuan). Such as figure 2 Show.
[0036] from figure 2 A can be seen that Aβ 42 Dense fibrous aggregates were formed after 48 hours of culture alone, figure 2 CTFBA and Aβ in B 42 After co-cultivation, ...
Embodiment 3
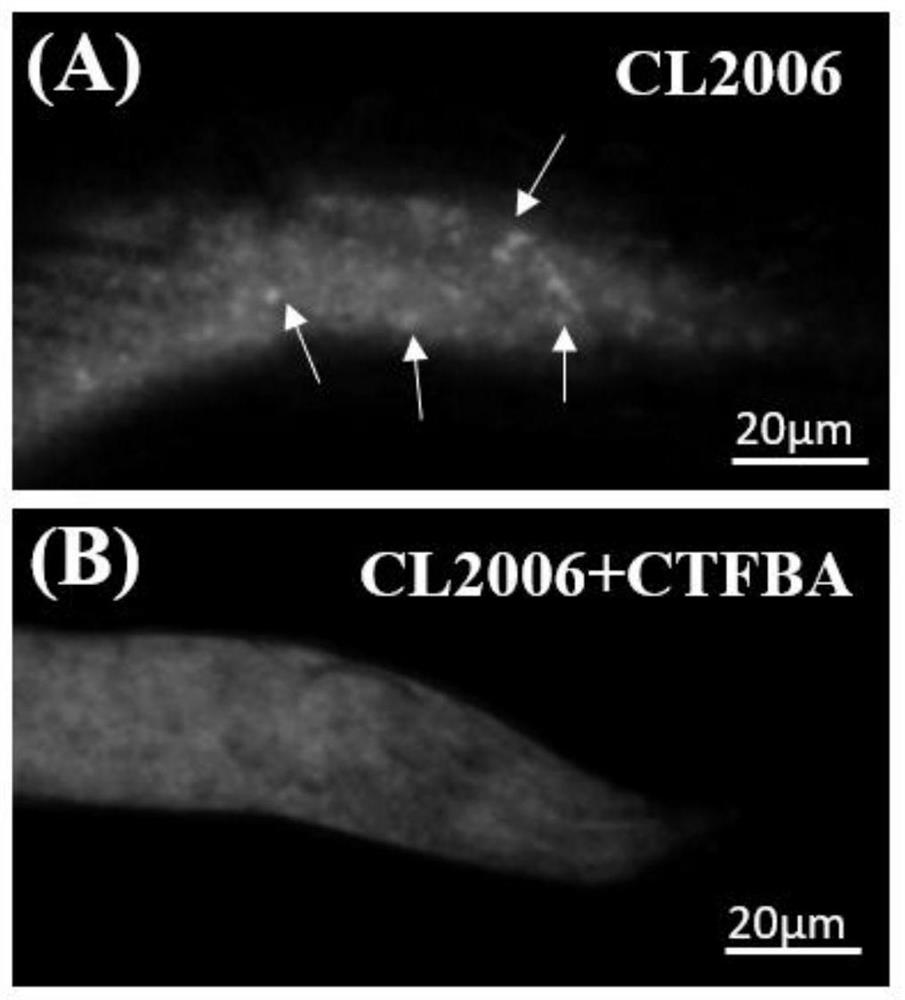
[0037] Example 3: The effect of CTFBA on the removal of amyloid plaques in Caenorhabditis elegans CL2006.
[0038] First prepare NMG medium, weigh 17g of agar powder, 2.5g of peptone and 3g of sodium chloride into the Erlenmeyer flask, add deionized water to 1L, sterilize at 120°C for 20 minutes, cool to 70°C, and pour Add 25mL of 1M potassium dihydrogen phosphate, 1mL of 1M magnesium sulfate, 1mL of 5mg / mL cholesterol and 1mL of 1M calcium chloride into the Erlenmeyer flask in sequence, shake well, pour into the plate, and dry it for later use.
[0039] Prepare LB liquid medium, weigh 2g of peptone, 1g of yeast powder and 2g of sodium chloride into the conical flask, add deionized water to 200mL, pick Escherichia coli OP50 into the LB liquid medium, at 37°C and 220rpm Placed on a shaker for 12 hours. Apply the cultured OP50 bacteria solution to the NGM medium, drop 200 μL into each plate, and put it upside down in a 4°C refrigerator after the bacteria solution is dried.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| P value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com