Tachyplesin I antibacterial peptide derivative as well as preparation method and application thereof
A technology of antibacterial peptides and derivatives, applied in the field of biomedicine, can solve the problem of limited sources of limulus, and achieve the effects of simple structure, wide bactericidal spectrum and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The synthetic method of above-mentioned antibacterial peptide derivative, comprises the following steps:
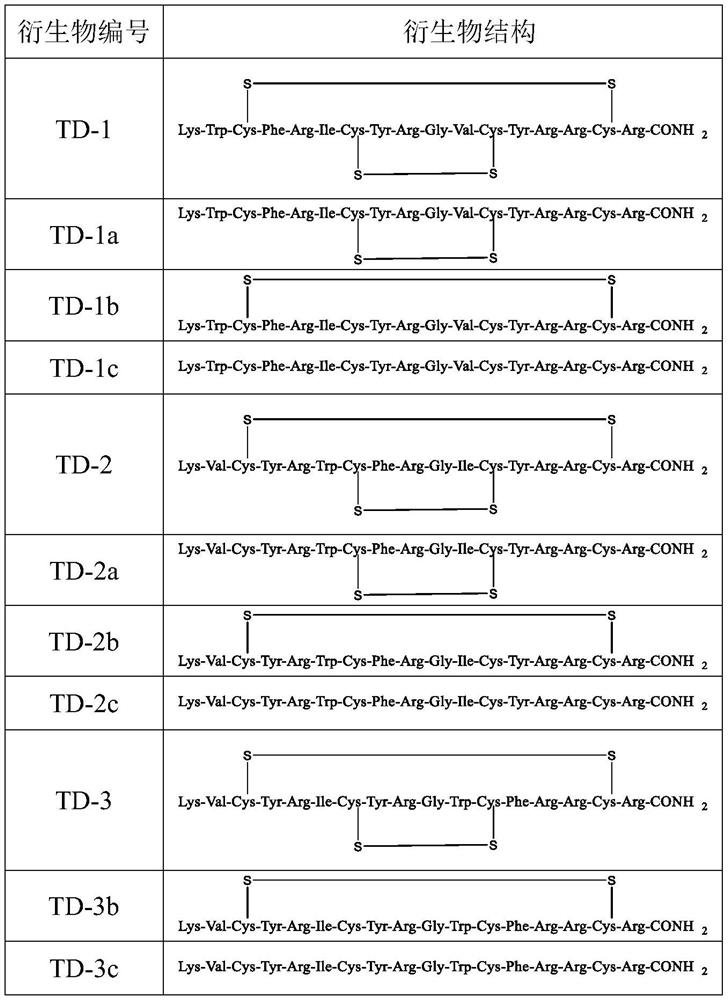
[0044] (1) Compounds TD-1c, TD-2c, TD-3c, TD-4c, and TD-5c without disulfide bonds were synthesized by solid-phase synthesis method, using Fmoc-amino resin as solid-phase carrier, and using polypeptide solid-phase The synthesis method is to sequentially couple the required amino acids from the C-terminal to the N-terminal according to the peptide sequence to obtain a linear fully protected peptide resin. Carry out cracking subsequently, lysate is selected the mixed solution of TFA, thioanisole, anisole and water volume ratio 90:5:2.5:2.5, and the amount of lysate is to use 8-20mL of lysate per 1g peptide resin, preferably 10mL, the lysis is carried out at room temperature, the lysis time is 1.5-3h, preferably 2h, after the lysis is completed, blow off excess trifluoroacetic acid with nitrogen, add glacial ether, a white solid is precipitated, the solid after centrifu...
Embodiment 1
[0049] Example 1: Synthesis of linear polypeptide TD-1c containing 0 pairs of disulfide bonds
[0050] Accurately weigh 500mg of Rink Amide resin (0.38mmol) with a degree of substitution of 0.76mmol / g in a solid-phase synthesis reactor, add DCM to swell for 20min, then add 20% piperidine / DMF solution twice to remove the Fmoc protecting group , remove for 5 minutes for the first time, remove for 20 minutes for the second time, wash with DMF, DCM and methanol for 3 to 5 times, and drain. Add pre-mixed and fully activated Fmoc-Arg(pbf)-OH (0.38mmol), HoBt (0.46mmol), DIC (0.46mmol), DMF (5ml) into the reactor, react at room temperature for 1h, wash with DMF for 3 ~5 times, drained, add 5mL of blocking reagent (acetic anhydride:DIPEA molar ratio is 1:1) into the reactor, react at room temperature for 2h, wash with DCM, methanol and DMF respectively for 3~5 times, repeat the above-mentioned Fmoc removal The removal step and the post-washing step, and then continue to add Fmoc-Trp(...
Embodiment 2
[0051] Example 2: Synthesis of linear polypeptide TD-1 containing 2 pairs of disulfide bonds
[0052] Accurately weigh 500mg of Rink Amide resin (0.38mmol) with a degree of substitution of 0.76mmol / g in a solid-phase synthesis reactor, add DCM to swell for 20min, then add 20% piperidine / DMF solution twice to remove the Fmoc protecting group , remove for 5 minutes for the first time, remove for 20 minutes for the second time, wash with DMF, DCM and methanol for 3 to 5 times, and drain. Add pre-mixed and fully dissolved Fmoc-Arg(pbf)-OH (0.38mmol), HoBt (0.46mmol), DIC (0.46mmol), DMF (5ml), react at room temperature for 1h, wash with DMF for 3 ~5 times, drained, add 5mL of blocking reagent (acetic anhydride:DIPEA molar ratio is 1:1) into the reactor, react at room temperature for 2h, wash with DCM, methanol and DMF respectively for 3~5 times, repeat the above-mentioned Fmoc removal The removal step and the post-washing step, and then continue to add Fmoc-Trp(Boc)-OH (0.38mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com