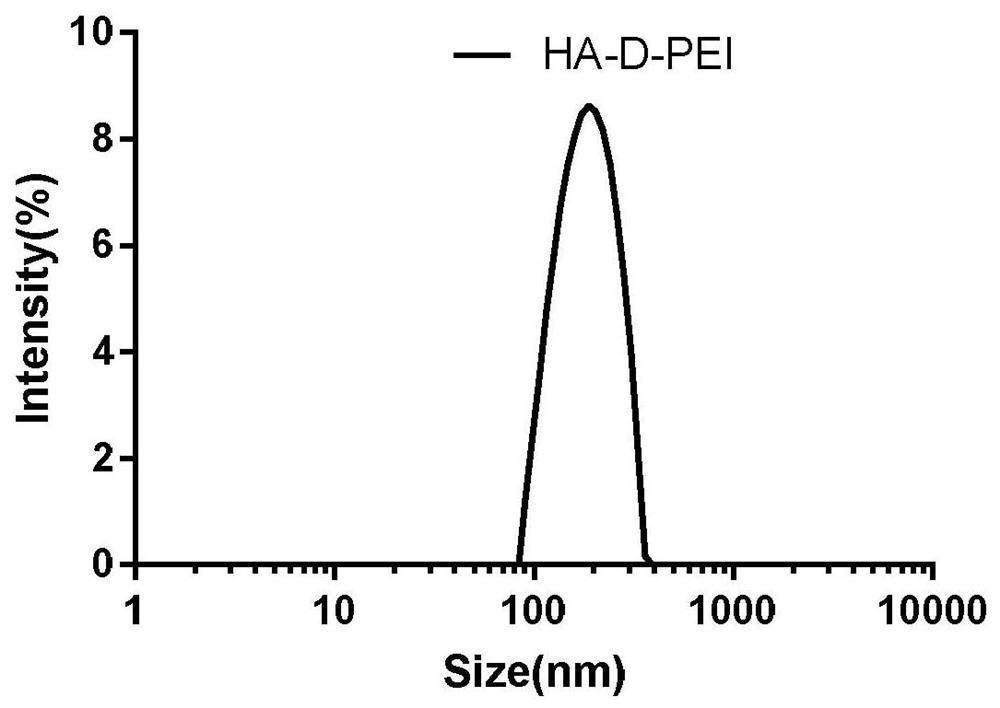
Preparation method and application of intelligent responsive shell-core polyelectrolyte nanogel
A nanogel and polyelectrolyte technology, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and capsule delivery, can solve problems such as inability to release quickly, low endocytosis efficiency, and premature ejaculation of drugs. , to increase the stability, efficient treatment effect, and improve the effect of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Synthesis of azide embodiment hyperbranched polyethyleneimine (D-PEI-N 3 )
[0034] (1) Synthesis of a hyperbranched polyethyleneimine (D-PEI)
[0035]
[0036] Take polyethyleneimine PEI (600mg, 1mmol) and N, N'- bis (acryloyl) cystamine (CBA, 260mg, 1mmol) were dissolved in 5mL methanol (MeOH), the mixture then was added 20μL triethylamine ( TEA), the reaction was stirred at room temperature. After the reaction, the reaction solution was collected by dialysis bag in MeOH dialysis, dialysis and then dialyzed medium was changed to high purity water, freeze-dried in vacuo to give the product.
[0037] (2) Synthesis of azide hyperbranched polyethyleneimine (D-PEI-N 3 )
[0038]
[0039] The azido acid (AATA, 12mg, 116μmol) was dissolved in water, was added 4- (4,6-dimethoxy-triazin-2-yl) -4-methylmorpholine hydrochloride (DMTMM, 25mg, 92.8 [mu] mol), stir at room temperature after the addition of the above-described hyperbranched polyethyleneimine (D-PEI, 50mg, ...
Embodiment 2
[0040] Example 2 Synthesis of active oxygen sensitive crosslinker embodiment RBCN
[0041]
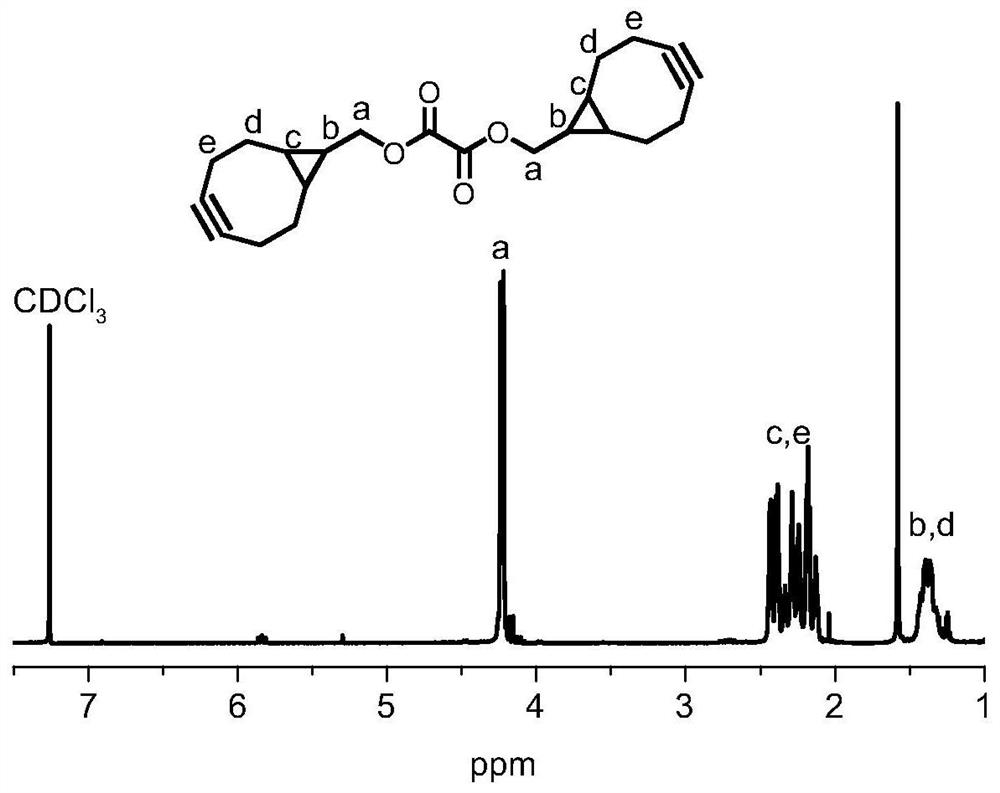
[0042]Double ring [6.1.0] non-4-alkyne-9-yl methanol (BCN, 100 mg, 0.67 mmol) was dissolved in DCM, 92 μl of TEA, nitrogen protection, slowly dropped grass acyl chloride under ice bath conditions (37 μL, 0.44 Mmol) The DCM solution was stirred at room temperature. After the reaction, the reaction solution was concentrated under reduced pressure, and the RBCN was isolated by column chromatography, and the product was concentrated under reduced pressure, and the white solid RBCN was obtained. Hydrogen magnetic spectrum figure 1 Indicated.
Embodiment 3
[0043] Example 3 Synthesis of azide hyaluronic acid (HA-N 3 )
[0044]
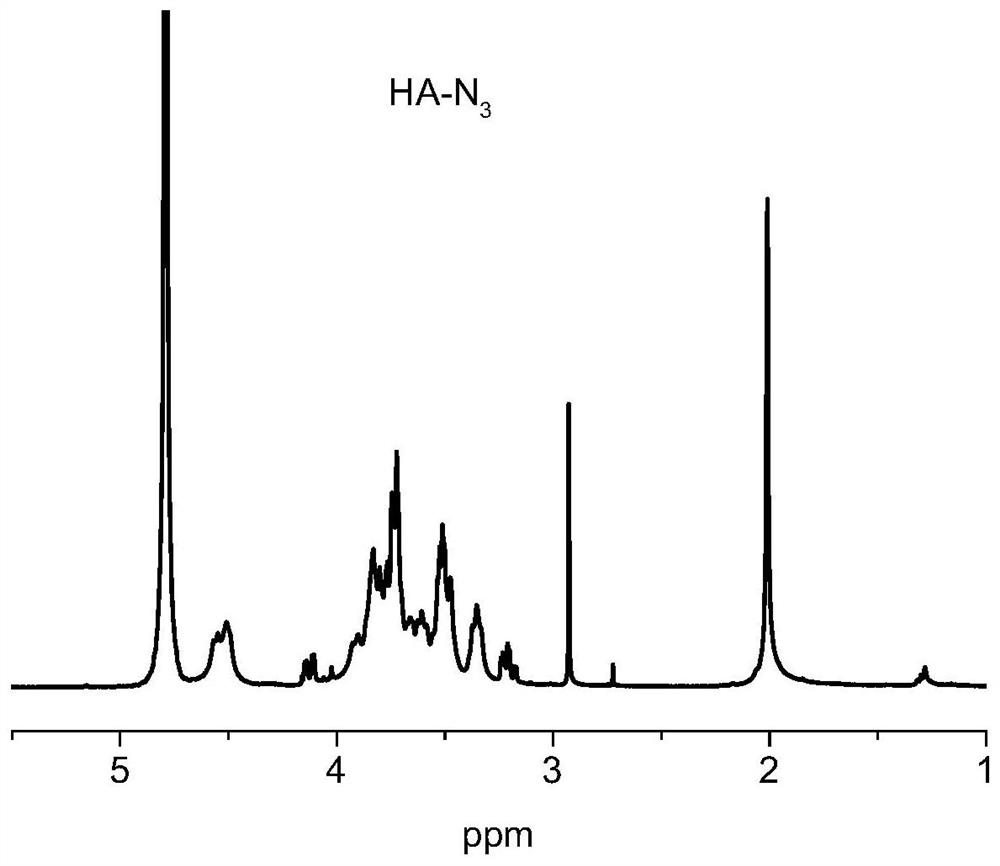
[0045] Hyaluronic acid (HA, 1G, 0.17 μmol) was dissolved in 20 mL of water, and DMTMM (110 mg, 0.4 mmol) was added. After stirring at room temperature, 2- [2- (2-azide ethoxy) ethoxy] Ethylamine (NH 2 -PEG 2 NN 3 (69 mg, 0.4 mmol), the pH was neutral, stirred at room temperature for 2 days, and the reaction liquid was collected in water after the reaction was completed, and the product was dried over frozen vacuum. Hydrogen magnetic spectrum figure 2 Indicated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com