Method for efficiently decomposing hydrogen peroxide by using graphene-coated cobalt catalyst under acidic condition
A technology of graphene coating and cobalt catalyst, which is applied in the field of materials chemistry, can solve the problems of increasing the difficulty of dissolution, separation and purification, recycling costs, oxidative damage of surface active groups, collapse of pore structure, etc., and achieves low raw material cost and good catalysis Performance, effect of preventing dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
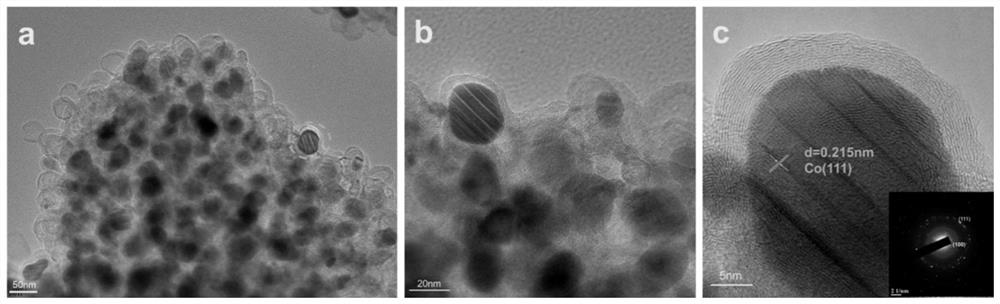
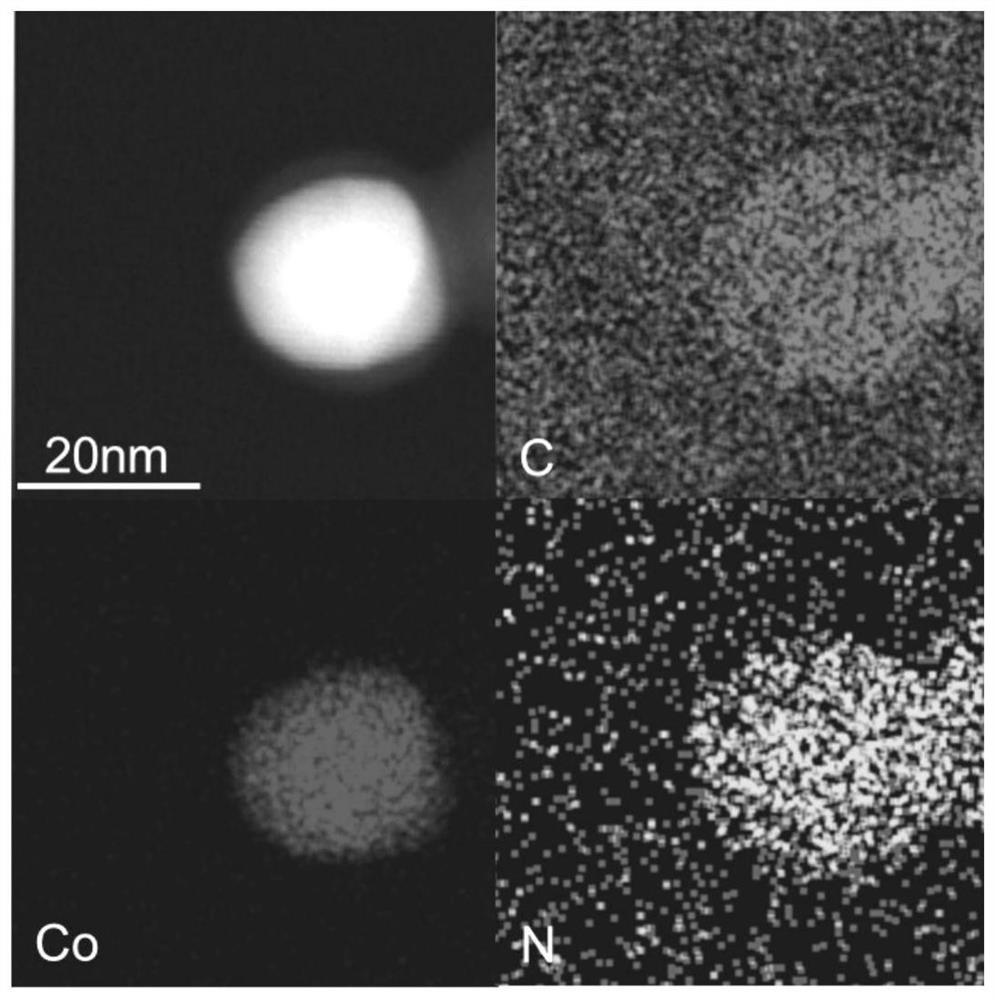
[0029] Weigh 291mg Co(NO 3 ) 2 .6H 2 The diaminomaleonitrile of 0 and 168.0mg is dissolved in 40mL dehydrated alcohol respectively, ultrasonic 30min makes it fully dissolve, then diaminomaleonitrile solution is poured in the hexahydrate cobalt nitrate solution, mixes and leaves standstill, after The mixed solution was heated at 135°C for 12 hours, cooled to room temperature, washed and dried, and the dried solid was ground into powder. The above powder was placed in a tube furnace under an Ar atmosphere, heated to 550 °C at a heating rate of 2 °C / min, and kept for 2 h. After being naturally cooled to room temperature, the resulting solid was dispersed in 2M H 2 SO 4, heated to 80°C and stirred for 2h, filtered while hot and washed 3 times with deionized water, dried the filter cake at 60°C for 12h, and the obtained solid was CoN 4 / C Catalyst.
[0030] CoN 4 Application of / C metal catalyst:
[0031] Get 10g hydrogen peroxide reaction solution (1M H 2 SO 4 ), of whic...
Embodiment 2
[0034] According to the catalyst preparation condition in embodiment 1, get the hydrogen peroxide reaction liquid (1M H 2 SO 4 ), of which about 10% wt is H 2 SO 4 and 1%wt H 2 o 2 , and the rest are water and trace impurities. In order to reduce the H in this product 2 o 2 content, the 40mg CoN 4 / C metal catalyst was added therein, stirred and reacted at room temperature for 2h, and the H in the sample was measured after the reaction was completed. 2 o 2 All decomposition, conversion rate 100%.
Embodiment 3
[0036] According to the catalyst preparation conditions in Example 1, get 10g of different acid concentrations (2~7M H 2 SO 4 ) hydrogen peroxide reaction solution, that is, about 20% wt to 70% wt is H 2 SO 4 and 1%wt H 2 o 2 , and the rest are water and trace impurities. In order to reduce the H in this product 2 o 2 content, the 40mg CoN 4 / C metal catalyst was added to it, stirred and reacted at 60°C for 1h, and the H in each sample was measured after the reaction 2 o 2 All decomposition, conversion rate 100%. It shows that the catalyst prepared by the present invention can efficiently decompose H under the environment of strong acid 2 o 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com