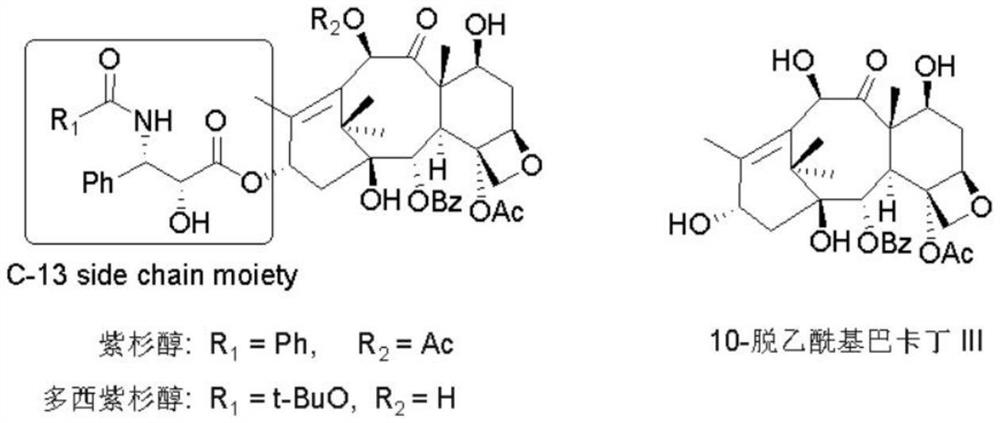
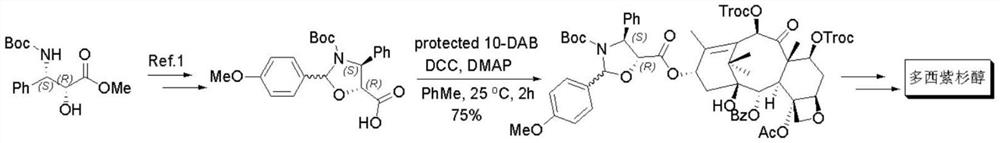
Preparation method of docetaxel chiral side chain intermediate
A technology of docetaxel and chiral side chain, applied in the field of drug synthesis, can solve the problems of long route, harsh reaction conditions, low total yield and the like, and achieves the effects of convenient route operation, good stereoselectivity, and simple separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
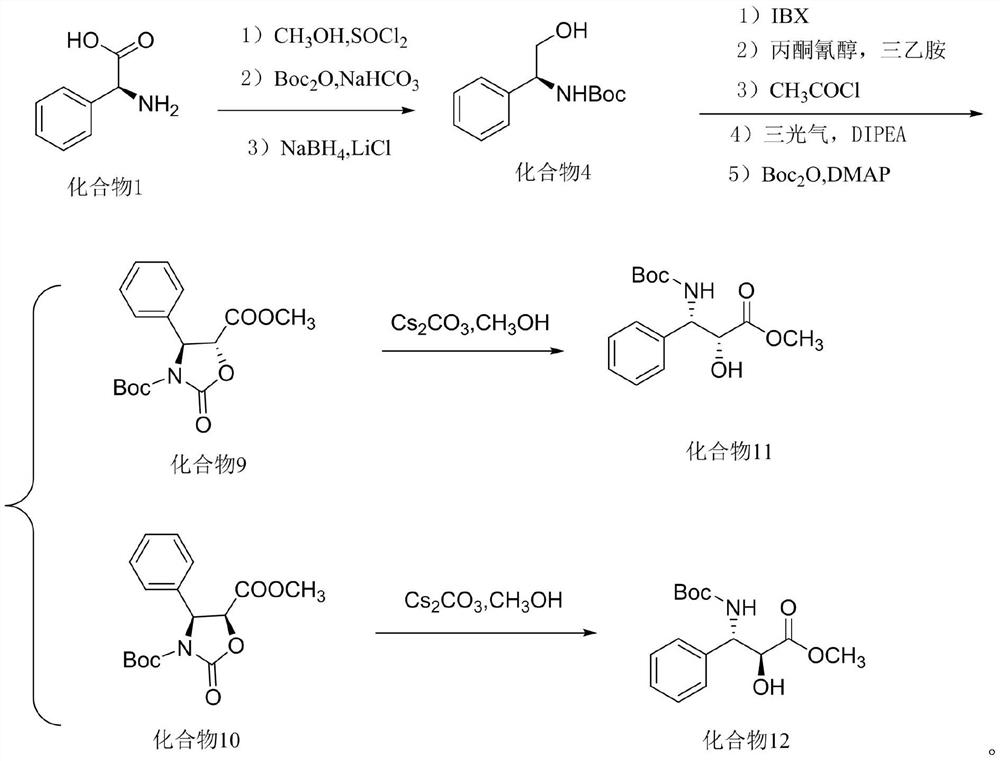
[0047] Example 1 Preparation of Compound 4
[0048] Dissolve 10.0g of compound I in 200mL of methanol solvent, slowly add 23.79g of thionyl chloride dropwise in an ice-water bath at 0°C, heat up to reflux for 3h after dropping for 30 minutes, and concentrate under reduced pressure after the reaction; add 400mL of tetrahydrofuran to the concentrated solution- Water (1:1, V / V) mixed solvent, add 16.80g of solid sodium bicarbonate, add 14.40g of Boc anhydride, react at room temperature for 10h, concentrate under reduced pressure after the reaction, add 300mL of purified water, add 250mL*3 ethyl acetate The ester was extracted 3 times, the organic phase was combined, washed with 200 mL of saturated brine, left to separate the liquid, the organic phase was dried by adding anhydrous sodium sulfate, filtered, and concentrated under reduced pressure; the concentrated solution was added with 600 mL of tetrahydrofuran-ethanol (1:1, V / V) Mixed solvent, add 7.57g of sodium borohydride an...
Embodiment 2
[0050] Example 2 Preparation of Compound 4
[0051] Dissolve 10.0g of compound I in 200mL of methanol solvent, slowly add 31.41g of thionyl chloride dropwise in an ice-water bath at 0°C, heat up to reflux for 3h after the drop is completed, and concentrate under reduced pressure after the reaction is complete; add 400mL of tetrahydrofuran to the concentrated solution- Water (1:1, V / V) mixed solvent, add 22.18g of solid sodium bicarbonate, add 14.69g of Boc anhydride, react at room temperature for 10h, concentrate under reduced pressure after the reaction, add 300mL of purified water, add 250mL*3 ethyl acetate The ester was extracted 3 times, the organic phase was combined, washed with 200 mL of saturated brine, left to separate the liquid, the organic phase was dried by adding anhydrous sodium sulfate, filtered, and concentrated under reduced pressure; the concentrated solution was added with 600 mL of tetrahydrofuran-ethanol (1:1, V / V) Mixed solvent, add 4.99g of sodium boro...
Embodiment 3
[0052] Example 3 Preparation of compound 4
[0053] Dissolve 10.0g of compound I in 200mL of methanol solvent, slowly add 39.26g of thionyl chloride dropwise in an ice-water bath at 0°C, heat up to reflux for 3h after the drop is completed, and concentrate under reduced pressure after the reaction is completed; add 400mL of tetrahydrofuran to the concentrated solution- Water (1:1, V / V) mixed solvent, add 27.72g of solid sodium bicarbonate, add 14.40g of Boc anhydride, react at room temperature for 10h, concentrate under reduced pressure after the reaction, add 300mL of purified water, add 250mL*3 ethyl acetate The ester was extracted 3 times, the organic phase was combined, washed with 200 mL of saturated brine, left to separate the liquid, the organic phase was dried by adding anhydrous sodium sulfate, filtered, and concentrated under reduced pressure; the concentrated solution was added with 600 mL of tetrahydrofuran-ethanol (1:1, V / V) Mixed solvent, add 9.99g of sodium bor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com