Method for preparing sodium ion battery positive electrode materials with different crystal forms through lithium doping regulation and control
A technology for sodium-ion batteries and cathode materials, which is applied in battery electrodes, secondary batteries, chemical instruments and methods, etc., can solve the problems of lack of controllable synthesis methods, and achieves a high degree of disorder, a simple process, and an increase in extra capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
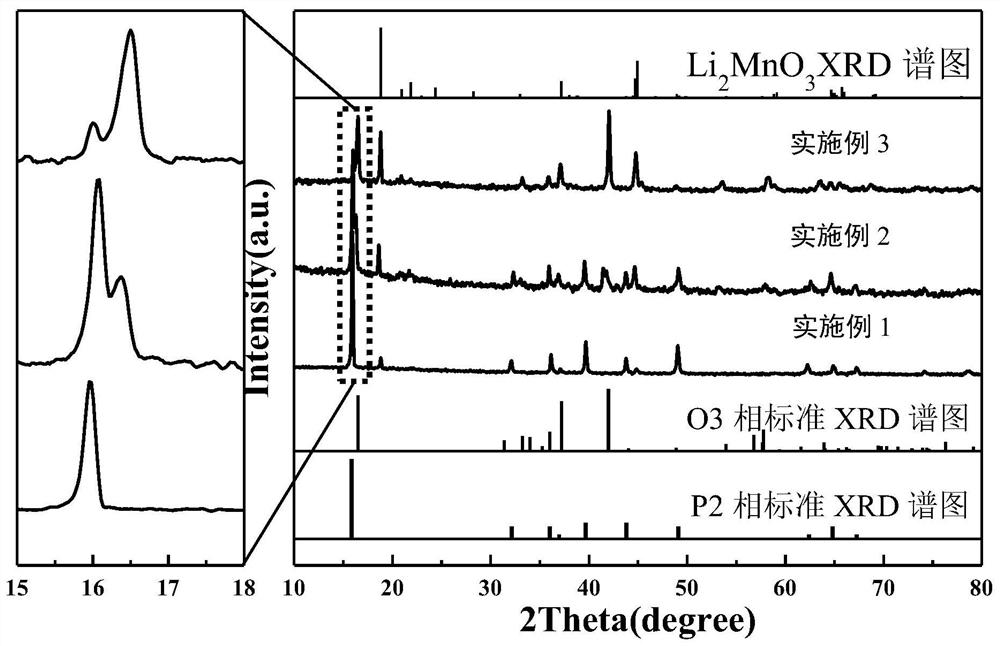
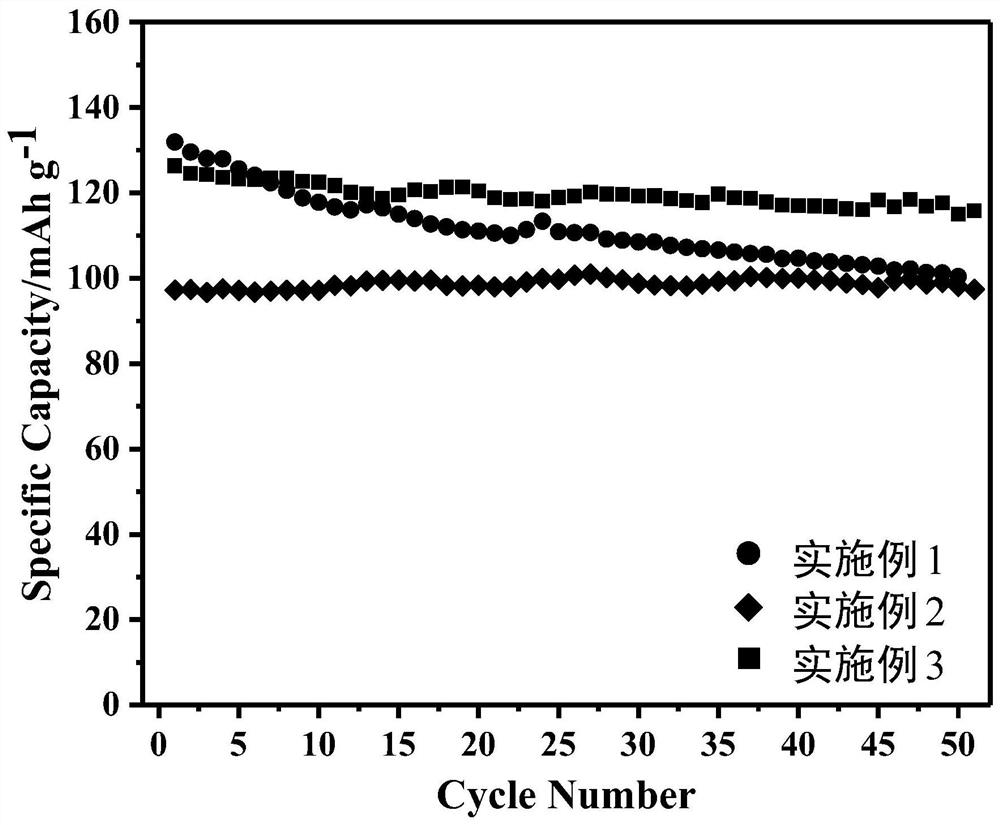
Embodiment 1
[0021] Embodiment 1: P2 type sodium ion battery Na 0.7 Ni 0.1 Fe 0.1 mn 0.6 Li 0.2 o 2 preparation of
[0022] (1) Dissolve 0.102g of lithium acetate, 0.735g of manganese acetate, 0.124g of nickel acetate and 0.202g of ferric nitrate in a mixture of 10mL of water and 50mL of ethanol to obtain a metal salt solution. Dissolve 0.946g of oxalic acid in a mixed solution of 10mL of water and 50mL of ethanol to obtain a precipitant solution. The precipitating agent solution was quickly poured into the metal salt solution and continued to stir for 6 hours, and the obtained suspension was dried in an oven at 80°C for 15 hours to obtain a precursor.
[0023] (2) Fully grind and mix the precursor with 0.185g of sodium carbonate, calcinate at 450°C for 4h in air atmosphere, and then calcinate at 800°C for 12h to obtain P2 type Na 0.7 Ni 0.1 Fe 0.1 mn 0.6 Li 0.2 o 2 Cathode material.
[0024] Mix the positive electrode material obtained in this example with acetylene black and...
Embodiment 2
[0025] Embodiment 2: P2 / O3 type sodium ion battery Na 0.7 Ni 0.1 Fe 0.1 mn 0.5 Li 0.3 o 2 preparation of
[0026] (1) Dissolve 0.153g of lithium acetate, 0.613g of manganese acetate, 0.124g of nickel acetate and 0.202g of ferric nitrate in a mixture of 10mL of water and 50mL of ethanol to obtain a metal salt solution. Dissolve 0.946g of oxalic acid in a mixed solution of 10mL of water and 50mL of ethanol to obtain a precipitant solution. The precipitating agent solution was quickly poured into the metal salt solution and continued to stir for 6 hours, and the obtained suspension was dried in an oven at 80°C for 15 hours to obtain a precursor.
[0027] (2) Fully grind and mix the precursor with 0.185g sodium carbonate, calcinate at 450°C for 4h in the air atmosphere, and then calcinate at 800°C for 12h to obtain P2 / O3 type Na 0.7 Ni 0.1 Fe 0.1 mn 0.5 Li 0.3 o 2 Cathode material.
[0028]Mix the positive electrode material obtained in this example with acetylene bla...
Embodiment 3
[0029] Embodiment 3: O3 type sodium ion battery Na 0.7 Ni 0.1 Fe 0.1 mn 0.4 Li 0.4 o 2 preparation of
[0030] (1) Dissolve 0.204g of lithium acetate, 0.490g of manganese acetate, 0.124g of nickel acetate and 0.202g of ferric nitrate in a mixture of 10mL of water and 50mL of ethanol to obtain a metal salt solution. Dissolve 0.946g of oxalic acid in a mixed solution of 10mL of water and 50mL of ethanol to obtain a precipitant solution. The precipitating agent solution was quickly poured into the metal salt solution and continued to stir for 6 hours, and the obtained suspension was dried in an oven at 80°C for 15 hours to obtain a precursor.
[0031] (2) Fully grind and mix the precursor with 0.185g of sodium carbonate, calcinate at 450°C for 4h in the air atmosphere, and then calcinate at 800°C for 12h to obtain O3 type Na 0.7 Ni 0.1 Fe 0.1 mn 0.4 Li 0.4 o 2 Cathode material.
[0032] Mix the positive electrode material obtained in this example with acetylene black...
PUM
| Property | Measurement | Unit |
|---|---|---|
| retention rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com