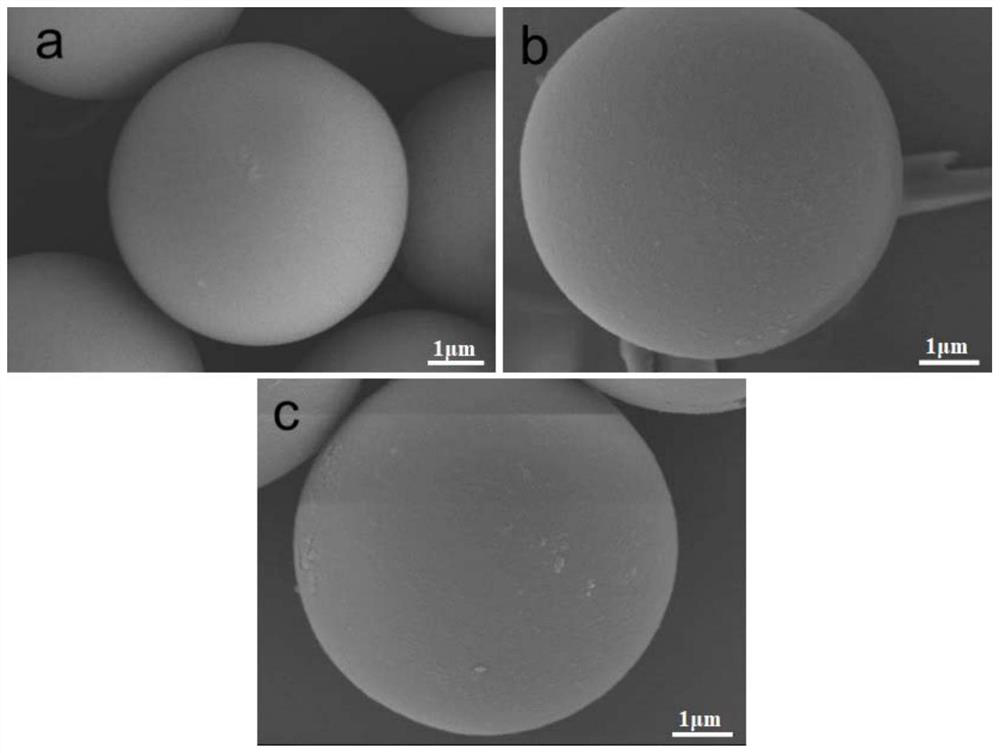
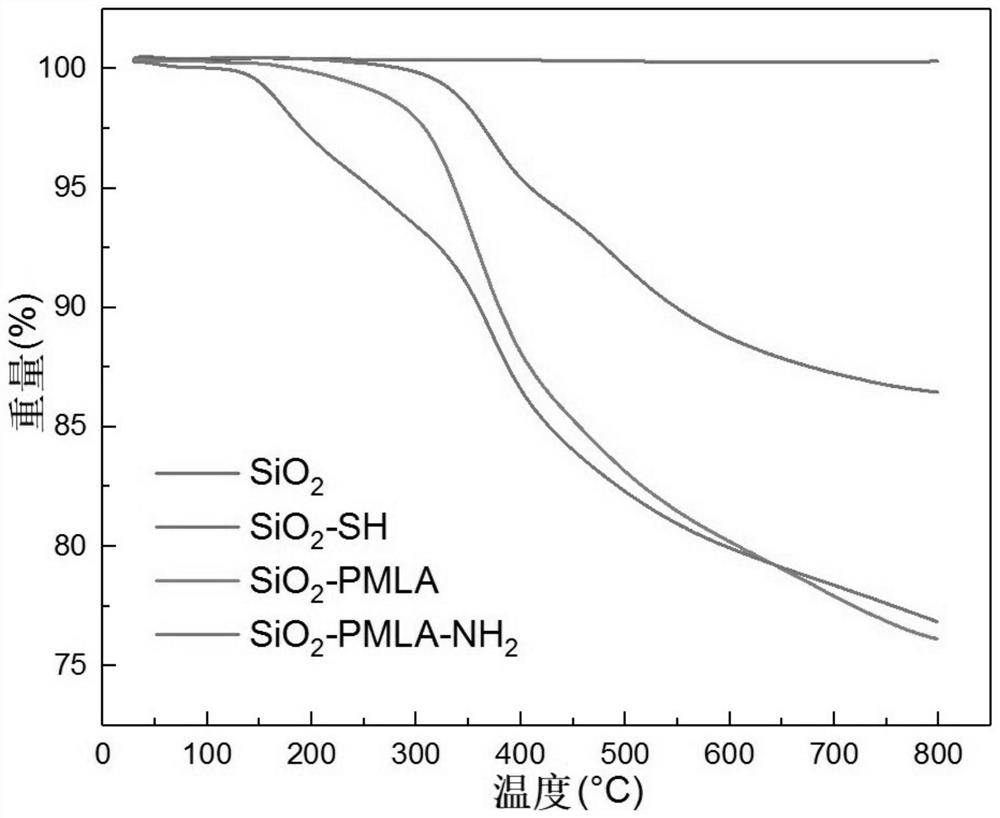
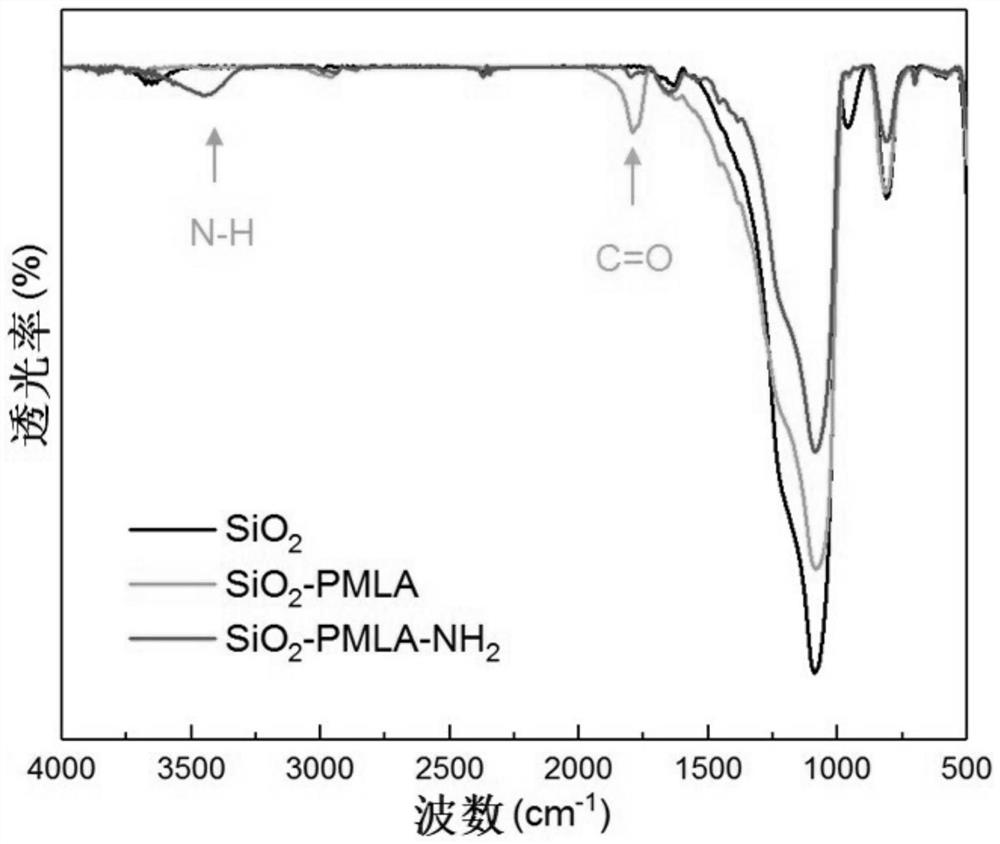
Lactide-derived chiral stationary phase as well as preparation method and application thereof
A technology of chiral stationary phase and lactide, which is applied in the field of high performance liquid chromatography, can solve the problems that chiral compounds cannot be separated, and achieve the effect of excellent separation performance, mild reaction conditions, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A chiral stationary phase derived from lactide, the preparation method of which comprises the following steps:
[0056] 1) Add 20g of L-lactide, 29.66g of N-bromosuccinimide and 300mL of toluene into a 500mL three-necked flask with a reflux condenser, heat the mixture to reflux under mechanical stirring, and then Disperse 1.14 g of azobisisobutyronitrile with 50 mL of toluene and drop it into a three-necked flask through a dropping funnel. After the addition, stir at 80°C for 20 h, cool the reaction solution to room temperature, filter, and evaporate the filtrate to dryness. The obtained solid (light yellow) was dissolved in dichloromethane, washed 3 times with saturated sodium bisulfite solution, washed 1 time with saturated NaCl solution, and the organic layer was washed with MgSO 4 After drying and evaporating to dryness, bromolactide was obtained (white crystals, yield 53%);
[0057] 2) Add 20.5g of brominated lactide and 200mL of dichloromethane into a three-nec...
Embodiment 2
[0063] A chiral stationary phase derived from lactide, the preparation method of which comprises the following steps:
[0064] The SiO of 3.5g embodiment 1 2 -PMLA was dispersed in 30mL of anhydrous N,N-dimethylformamide (DMF), and then 18mL of (R)-(+)-1-phenylethylamine was added dropwise, and after the addition was completed, it was reacted at room temperature for 48h and filtered. The filtered solid was washed 3 times with acetonitrile, and dried under vacuum at 60°C for 15 h to obtain the lactide-derived chiral stationary phase SiO 2 -PMLA-NH 2 .
[0065] SiO 2 -PMLA-NH 2 The synthetic reaction of is as follows:
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com