Application of syringaresinol in preparation of medicine for preventing and treating diabetic nephropathy
A technology for diabetic nephropathy and syringaresinol, which is applied in the fields of drug combination, urinary system diseases, food science, etc., can solve the problem that there is no syringaresinol for diabetic nephropathy, and achieves the expansion of new uses, improvement of kidney damage, and less toxic and side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
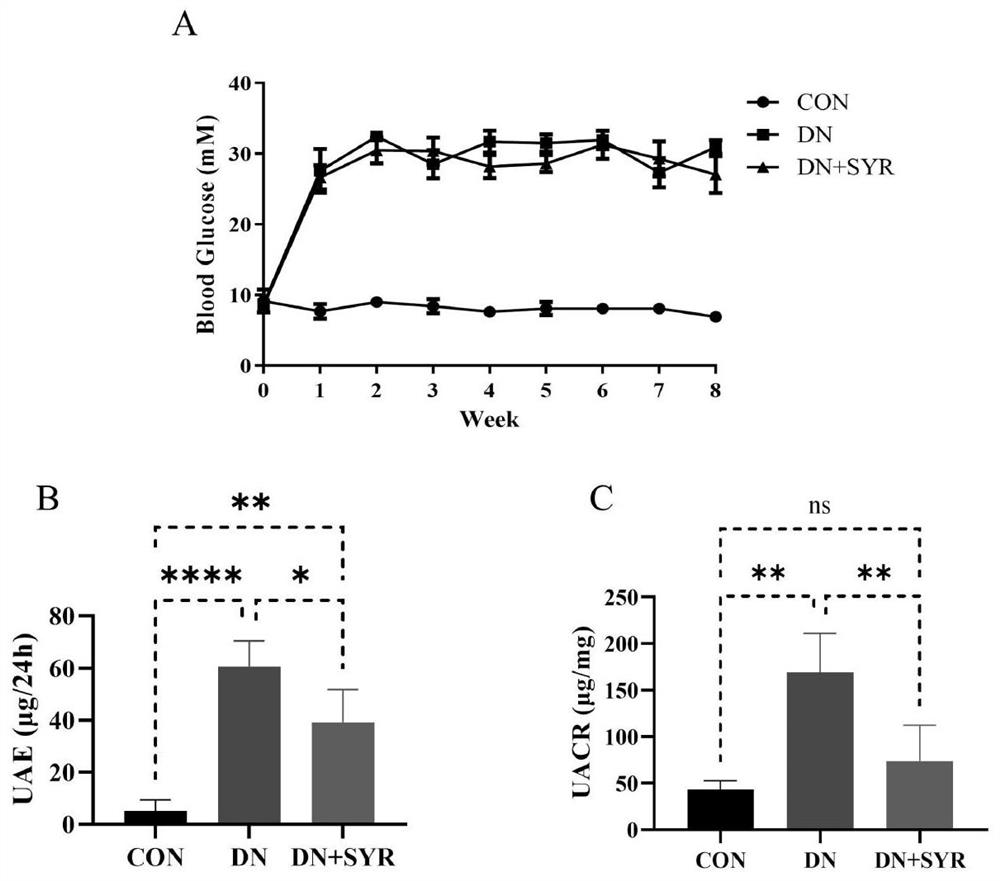
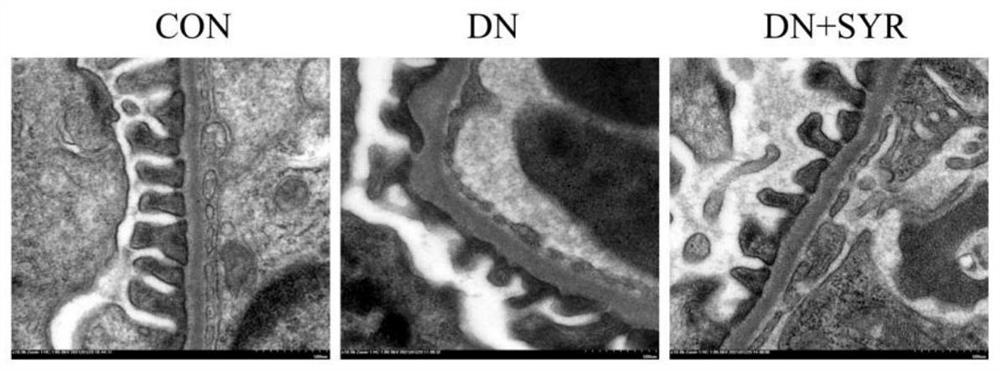
[0020] Embodiment one: the detection of blood sugar, urine albumin and urine creatinine, appended figure 1 The test results of blood glucose, urine albumin and urine creatinine in mice are given.
[0021] Randomly divide 24 male C57BL / 6 mice aged 6-8 weeks into the control group (CON group, 8) and the model group (16); Inject STZ intraperitoneally; 1 week after STZ injection, cut off the tip of the tail of the mouse to collect blood from the tail vein to detect the blood glucose level of the mouse. If the blood glucose > 11.4mM, the diabetic mice were successfully modeled; the diabetic mice were randomly divided into the model group (DCM group, 8) and drug treatment group (DCM+SYR group, 8); the mice in the drug treatment group were treated by intragastric administration of 25 mg / kg dose of SYR for 8 weeks, and the mice in other groups were treated by intragastric administration of normal saline at the same dose as a control. And the blood glucose of mice in each group was de...
Embodiment 2
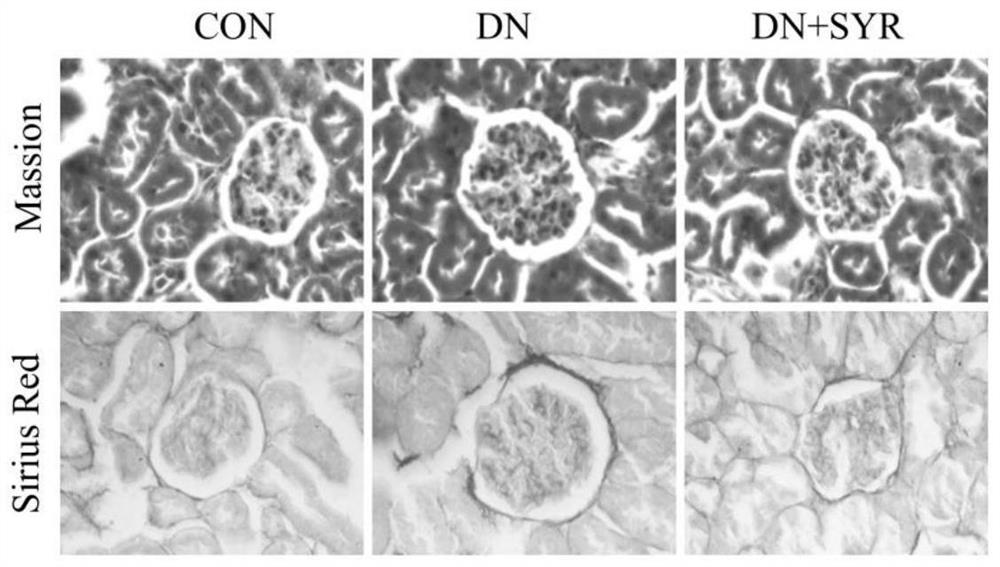
[0023] Example 2: Masson staining of kidney tissue
[0024] Soak the trimmed kidney tissue in 4% paraformaldehyde, fix at room temperature for more than 24 hours, perform gradient dehydration and embedding, and make 6 μm tissue sections; after dewaxing and rehydration, hematoxylin staining for 5-10 minutes; acidic ethanol differentiation 5-15s; the bluing solution turns blue for 3-5min; ponceau staining for 5-10min; dehydrated and transparent; figure 2 .
Embodiment 3
[0025] Example 3: Sirius Scarlet Staining of Kidney Tissue
[0026] Soak the trimmed kidney tissue in 4% paraformaldehyde, fix at room temperature for more than 24 hours, perform gradient dehydration and embedding, and make 6 μm tissue sections; after dewaxing and rehydration, stain with Sirius red staining solution for 1 hour; hematoxylin stains cell nuclei 8-10min; conventional dehydration and transparency; neutral gum sealing; observation and photography under a 400-fold microscope, see attached figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com