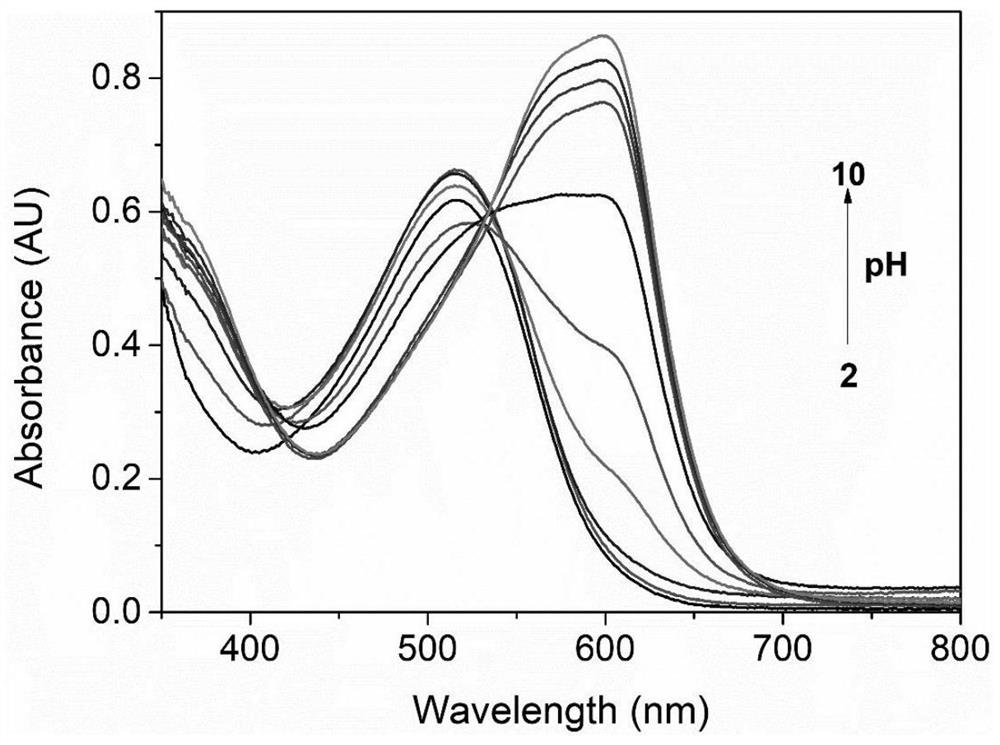
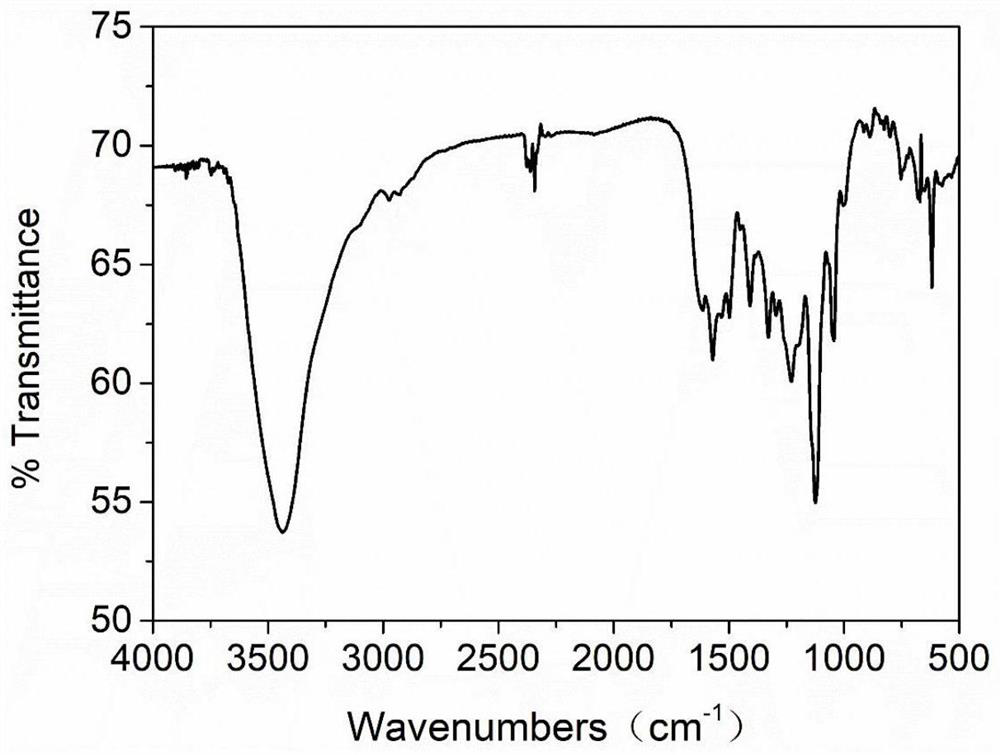
Benzothiazole heterocyclic azo type water-soluble dye and preparation method thereof
A technology for heterocyclic azo-type, water-soluble dyes, applied in the field of dyes, to achieve the effects of broad application prospects, high sensitivity, and excellent acid-base discoloration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
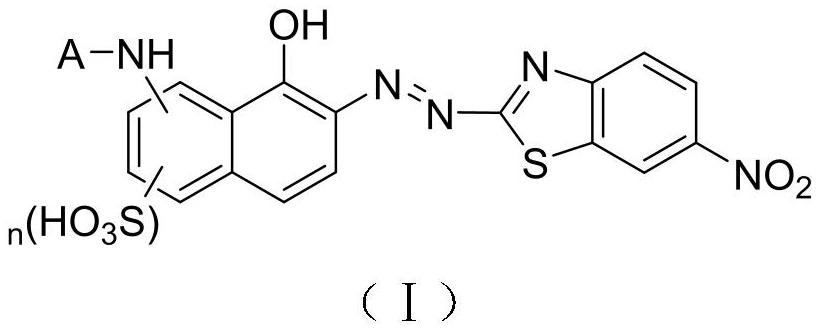
[0047] A benzothiazole heterocyclic azo type water-soluble dye, the specific structure is as follows:
[0048]
[0049] A method for preparing the above-mentioned benzothiazole heterocyclic azo type water-soluble dye:
[0050] Diazotization reaction: After cooling 11.0g of concentrated sulfuric acid with a mass fraction of 98% in an ice bath at 0-5°C, gradually add 0.73g of solid sodium nitrite, stir, and heat until completely dissolved, and the solution becomes clear and transparent , stop heating and cooling, gradually add 1.99g of 2-amino-6-nitrobenzothiazole (0.01mol, 98%), stir, then continue the reaction at 0~5°C, and detect the end point of the reaction by thin layer chromatography (TLC). After the reaction is completed, add a small amount of sulfamic acid to remove excess nitrous acid;
[0051] Condensation reaction of cyanuric chloride: take 1.90g cyanuric chloride (0.0103mol) and 20g small ice cubes, mechanically stir until milky at 0-5°C, then add 4.0g (0.01mol, 8...
Embodiment 2
[0056] A benzothiazole heterocyclic azo type water-soluble dye, the specific structure is as follows:
[0057]
[0058] A method for preparing the above-mentioned benzothiazole heterocyclic azo type water-soluble dye:
[0059] Diazotization reaction: same as the preparation in Example 1;
[0060] Condensation reaction of cyanuric chloride: take 1.90g cyanuric chloride (0.0103mol) and 20g small ice cubes, mechanically stir until milky at 0-5°C, then add 4.0g (0.01mol, 85.6%) H acid mono The aqueous solution of sodium salt, the solution adjusts pH between 4~5, detects and determines reaction end point with Ehrlich reagent; After reaction finishes, add 2.81g between (beta-sulfate ethyl sulfone) aniline (0.01mol), will The temperature is raised to 20-30°C, and the pH value is controlled between 5-6 with 10% sodium carbonate solution. After 3-4 hours of reaction, TLC detects the reaction end point (developing agent is n-butanol: isopropanol: ethyl acetate ester:water=2:4:1:3, ...
Embodiment 3
[0063] A benzothiazole heterocyclic azo type water-soluble dye, the specific structure is as follows:
[0064]
[0065] A method for preparing the above-mentioned benzothiazole heterocyclic azo type water-soluble dye:
[0066] Diazotization reaction: same as the preparation in Example 1;
[0067] Condensation reaction of cyanuric chloride: take 1.90g cyanuric chloride (0.0103mol) and 20g small ice cubes, mechanically stir until milky at 0-5°C, then add 4.0g (0.01mol, 85.6%) H acid mono An aqueous solution of sodium salt, the pH of the solution is adjusted between 4 and 5, and the end point of the reaction is determined by Ehrlich reagent detection; after the reaction is finished, add 1.77g of anthranilic acid (0.01mol, 98%) to raise the temperature To 20~30℃, use 10% sodium carbonate solution to control the pH value between 5~6, after reacting for 3~4 hours, TLC detects the reaction end point (developing agent is n-butanol: isopropanol: ethyl acetate: water =2:4:1:3, v / v)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com