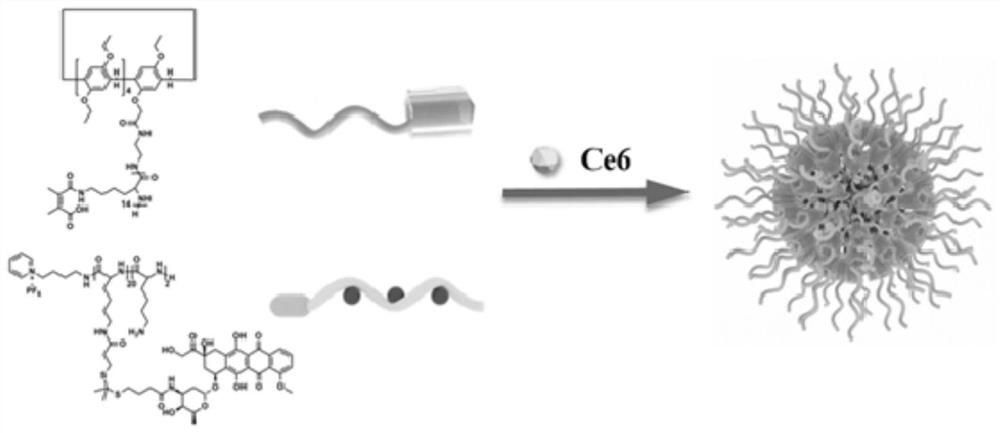
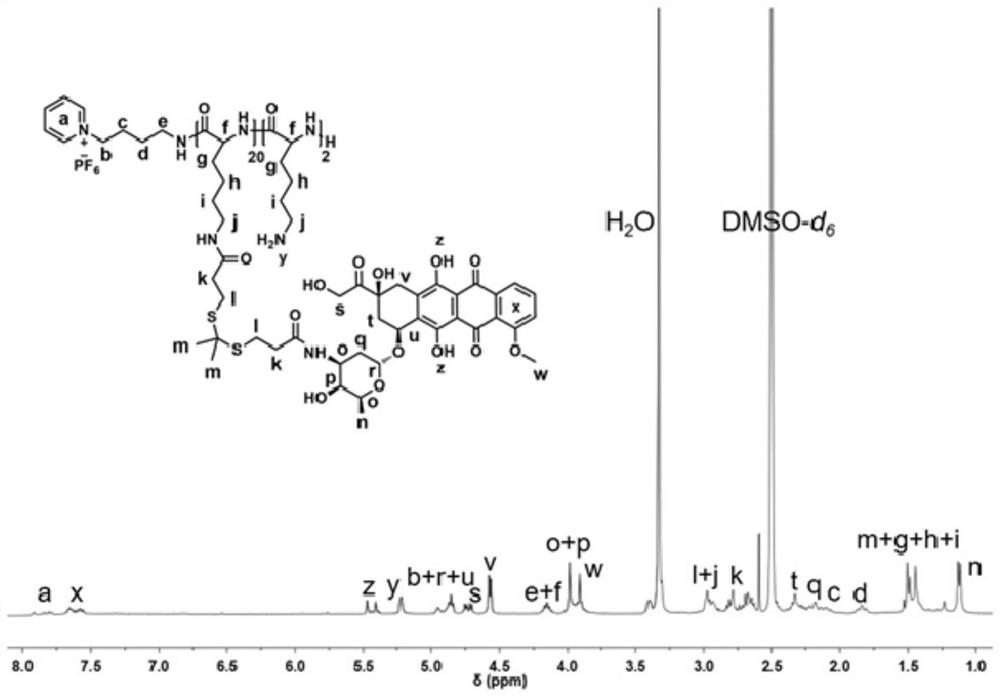
Charge reversal type supramolecular polypeptide prodrug nanoparticles as well as preparation method and application thereof
A charge inversion, nanoparticle technology, applied in the field of biomedicine, can solve problems such as low drug loading efficiency, drug leakage, and nano-drug instability, and achieve the effects of enhanced interaction, improved uptake, and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
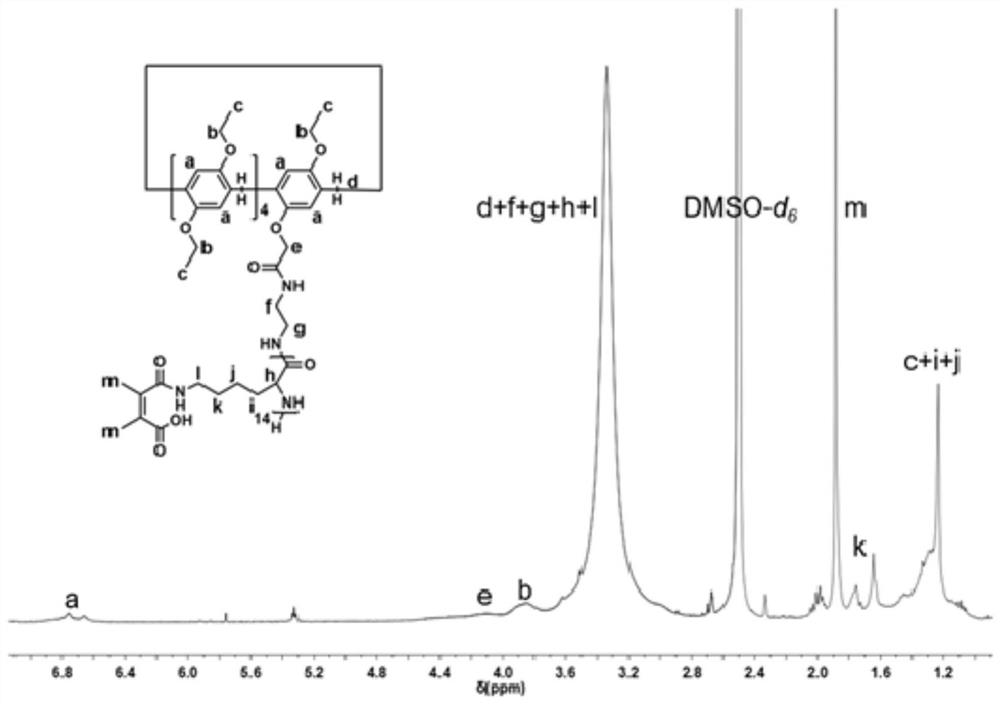
[0032] 1. Pillar[5]arene-poly( L - the preparation of lysine)
[0033] Step 1: Refer to the existing literature to obtain monoaminopillar[5]arene and ε-benzyloxycarbonyl- L -Lysine anhydride, in a glove box, take monoaminopyr[5]arene (0.033mmol, 31.76mg), dissolve it in 2mL of anhydrous N,N-dimethylformamide, and then add ε-benzyloxycarbonyl - L -Lysine anhydride (0.68mmol, 200mg), after reacting at room temperature for 48h, the reaction solution was settled in 16mL of anhydrous ether, centrifuged again, repeated 3 times, and vacuum dried for 24h to obtain 124.2mg of white solid. The yield is 82.3-84.3%.
[0034]
[0035]Step 2: Dissolve the white solid (0.0224mmol, 100mg) obtained in Step 1 in a mixed solvent of 10mL glacial acetic acid / trifluoroacetic acid (1:1 by volume), and add 1.1mL hydrobromic acid / ice at 0°C Acetic acid (33wt%) mixed solution was continued for 1.5h. After the reaction, the reaction solution was settled in 80 mL of anhydrous ether, centrifuged a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com