Method and system for removing fluorine and/or chlorine from sulfate solution through flash evaporation
A sulphate and flash evaporation technology, which is applied in chemical instruments and methods, flash evaporation, copper sulfate, etc., can solve problems such as the difficulty in realizing the resource utilization of fluorine and chlorine, the difficulty in meeting the requirements of the hydrometallurgy system, and the inability to achieve simultaneous removal, etc. Achieve the effect of environmental friendliness, less investment in equipment, and low cost of removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
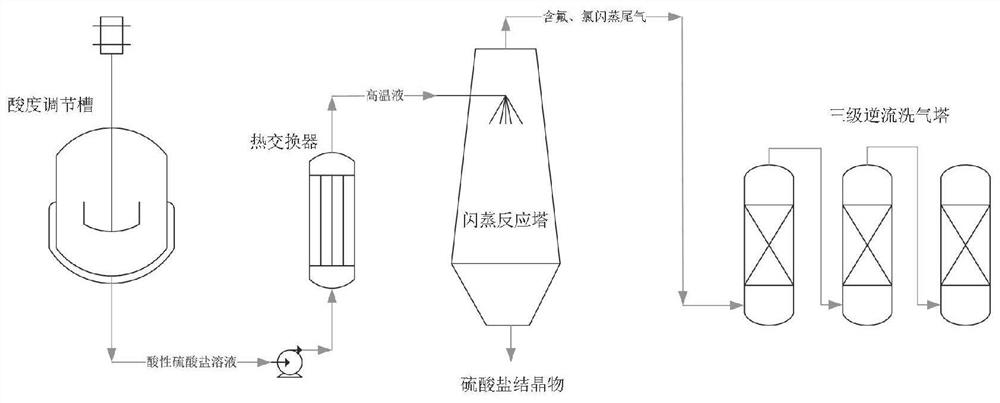
[0046] Taking the removal of fluorine and chlorine in copper sulfate solution (Cu:84.4g / L; F:127.2mg / L; Cl:3.9g / L, pH=4.2) as an example, first add sulfuric acid to the copper sulfate solution to adjust the acidity of the solution (Referring to the concentration of sulfuric acid in the mixed solution) is 13.1g / L, after mixing evenly, pump it into the high-pressure plate heat exchanger with a high-pressure pump, control the flow rate to 4.2L / min, and ensure that the temperature of the copper sulfate solution at the outlet is 180±3 ℃, and then introduced into the preheated flash evaporation chamber. When performing flash evaporation, it is necessary to maintain the temperature of the volatilization chamber at 120°C and the pressure at 600-800Pa. After the flash evaporation is completed, copper sulfate crystalline slag can be obtained at the bottom of the flash reactor, and the crystalline slag is collected and sampled for analysis of fluorine and chlorine content. The fluorine ...
Embodiment 2
[0052] Taking the removal of fluorine and chlorine in zinc sulfate solution (Zn: 162.7g / L; F: 1207.8mg / L; Cl: 3.1g / L, pH=4.6) as an example, first add sulfuric acid to the copper sulfate solution to adjust the acidity of the solution After mixing evenly, pump it into the high-pressure plate heat exchanger with a high-pressure pump, control the flow rate to 4.5L / min, ensure that the temperature of the copper sulfate solution at the outlet is 210±3°C, and then introduce it into the preheated flash evaporation chamber. When performing flash evaporation, it is necessary to maintain the temperature of the volatilization chamber at 130°C and the pressure at 300-500Pa. After the flash evaporation is completed, zinc sulfate crystalline slag can be obtained at the bottom of the flash reactor, and the crystalline slag is collected and sampled for analysis of fluorine and chlorine content. The fluorine content in the zinc sulfate crystal slag is less than 1ppm, and the chlorine content ...
Embodiment 3
[0058] Taking the removal of fluorine and chlorine in nickel sulfate solution (Ni:92.4g / L; F:2415.4mg / L; Cl:1.4g / L, pH=2.5) as an example, first add sulfuric acid to the copper sulfate solution to adjust the acidity of the solution After mixing evenly, pump it into the high-pressure plate heat exchanger with a high-pressure pump, control the flow rate to 2.7L / min, ensure that the temperature of the copper sulfate solution at the outlet is 180±3°C, and then introduce it into the preheated flash evaporation chamber. When performing flash evaporation, it is necessary to maintain the temperature of the volatilization chamber at 120°C and the pressure at 400-600Pa. After the flash evaporation is completed, nickel sulfate crystalline slag can be obtained at the bottom of the flash reactor, and the crystalline slag is collected and sampled for analysis of fluorine and chlorine content. The fluorine content in the nickel sulfate crystal slag is less than 1ppm, and the chlorine conten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com