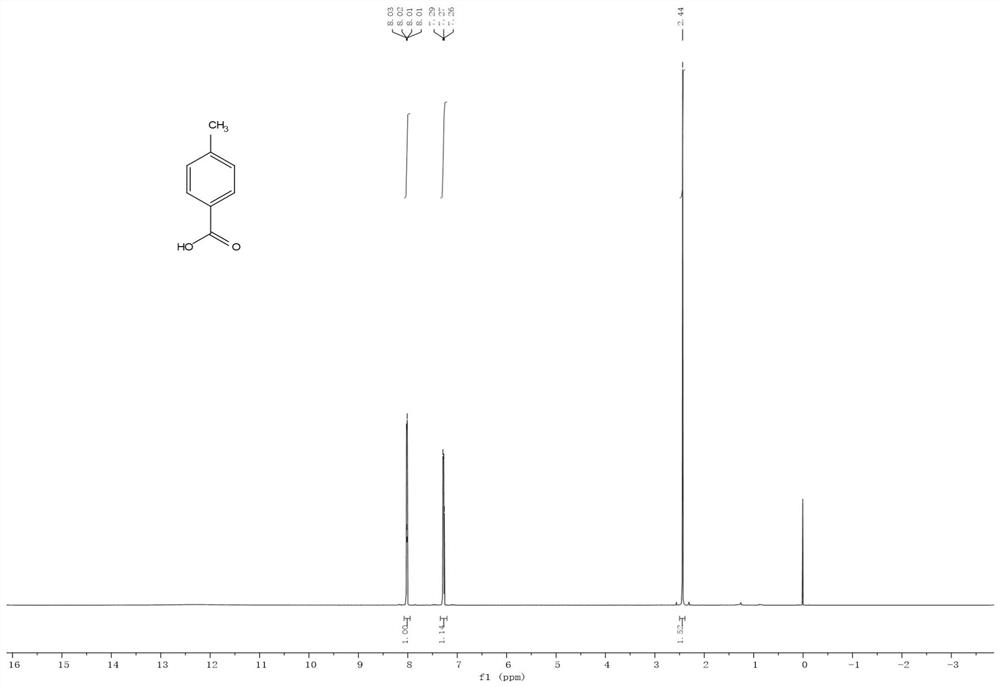
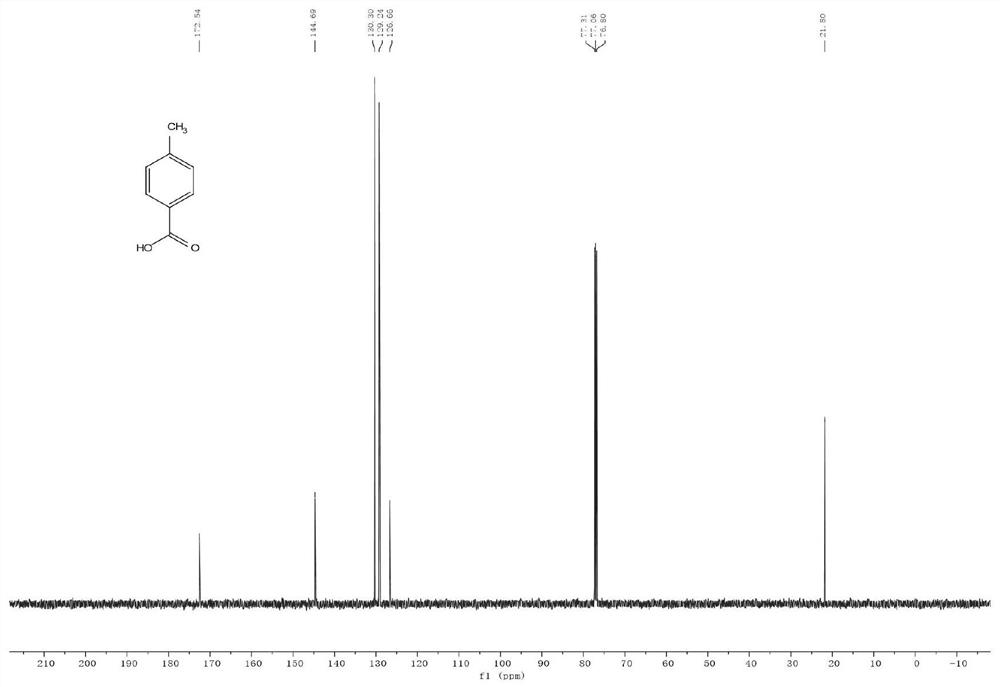
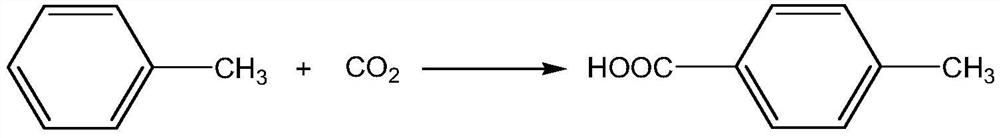
Method for preparing p-toluic acid by catalyzing carbon dioxide and methylbenzene
A technology of p-toluic acid and carbon dioxide, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve the problems of poor selectivity, high cost and high cost of industrial preparation, and improve stability and Activity, enhanced atom utilization, and mild effects of pressure conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In an intelligent reactor equipped with 3.5g toluene, 0.67g 50% aluminum trichloride BEA zeolite solid carrier and 0.50g 1H, 1H, 2H, 2H-perfluorooctyltrichlorosilane, carbon dioxide was introduced under magnetic stirring, Control the temperature of 40°C and the pressure of 3.0MPa to react for 8h. After the reaction, dilute hydrochloric acid was added to the reaction mixture to adjust the pH to 6-7, then 10.0 g of dichloromethane was added, the organic phase was separated by solid-liquid and liquid-liquid separation, and the organic phase was further washed with pure water until the organic phase was neutral, and finally the organic phase was washed by liquid The organic phase was concentrated by liquid separation and evaporation under reduced pressure, and crystalline p-toluic acid was purified by column chromatography with a yield of 66.5%.
Embodiment 2
[0033] In a reactor containing 3.5g of toluene, 0.13g of aluminum trichloride, 0.15g of BEA zeolite and 0.29g of triphenylchlorosilane, carbon dioxide was introduced under magnetic stirring, and the reaction was carried out at a temperature of 40°C and a pressure of 3.0MPa for 8h. After the reaction, dilute hydrochloric acid was added to the reaction mixture to adjust the pH to 6-7, then 10.0 g of dichloromethane was added, the organic phase was separated by solid-liquid and liquid-liquid separation, and the organic phase was further washed with pure water until the organic phase was neutral, and finally the organic phase was washed by liquid The organic phase was concentrated by liquid separation and evaporation under reduced pressure, and crystalline p-toluic acid was purified by column chromatography with a yield of 63.5%.
Embodiment 3
[0035] In the reactor equipped with 5.0mL of dichloromethane, add 0.92g of toluene, 0.50g of 50% aluminum trichloride BEA zeolite solid carrier and 0.50g of triphenylchlorosilane in the smart reactor, and feed carbon dioxide under magnetic stirring , Control the temperature of 40°C and the pressure of 5.0MPa to react for 8h. After the reaction, dilute hydrochloric acid was added to the reaction mixture to adjust the pH to 6-7, then 10.0 g of dichloromethane was added, the organic phase was separated by solid-liquid and liquid-liquid separation, and the organic phase was further washed with pure water until the organic phase was neutral, and finally the organic phase was washed by liquid The organic phase was concentrated by liquid separation and evaporation under reduced pressure, and crystalline p-toluic acid was purified by column chromatography with a yield of 40.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com