D-benzothiadiazole-TB(-D) derivative as well as synthesis method and application thereof
A technology of benzothiadiazole and synthesis method, which is applied in the directions of drug combinations, chemical instruments and methods, and medical preparations containing active ingredients, etc. problem, to achieve the effects of excellent solid-state luminescence, excellent luminescence performance, and wide variety of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
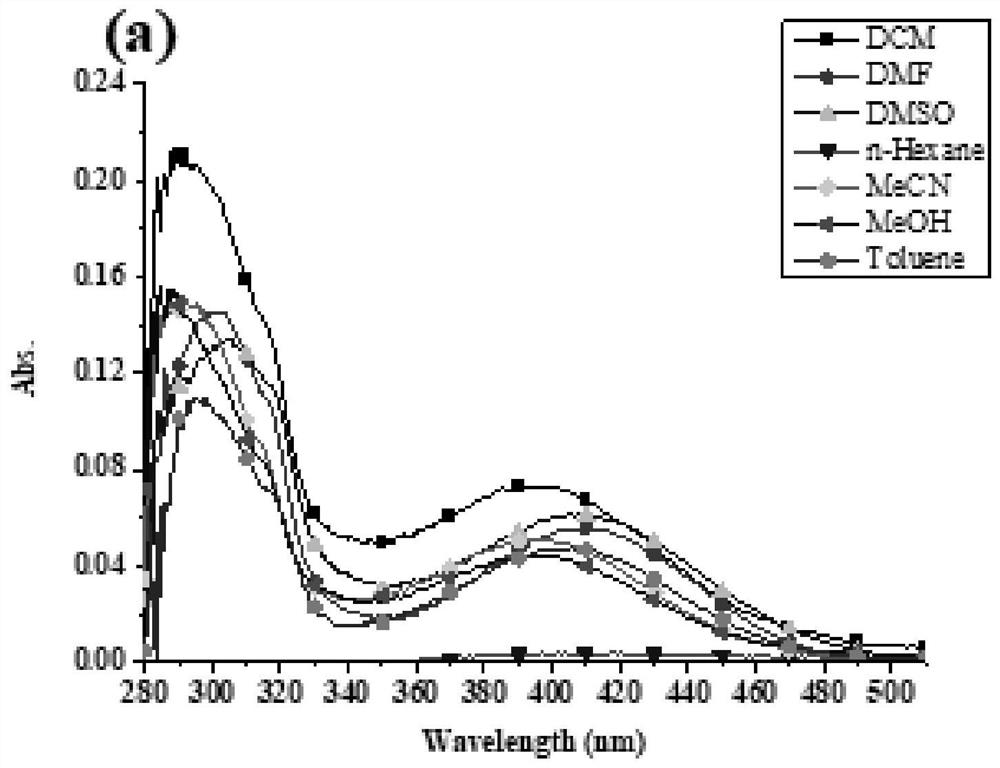
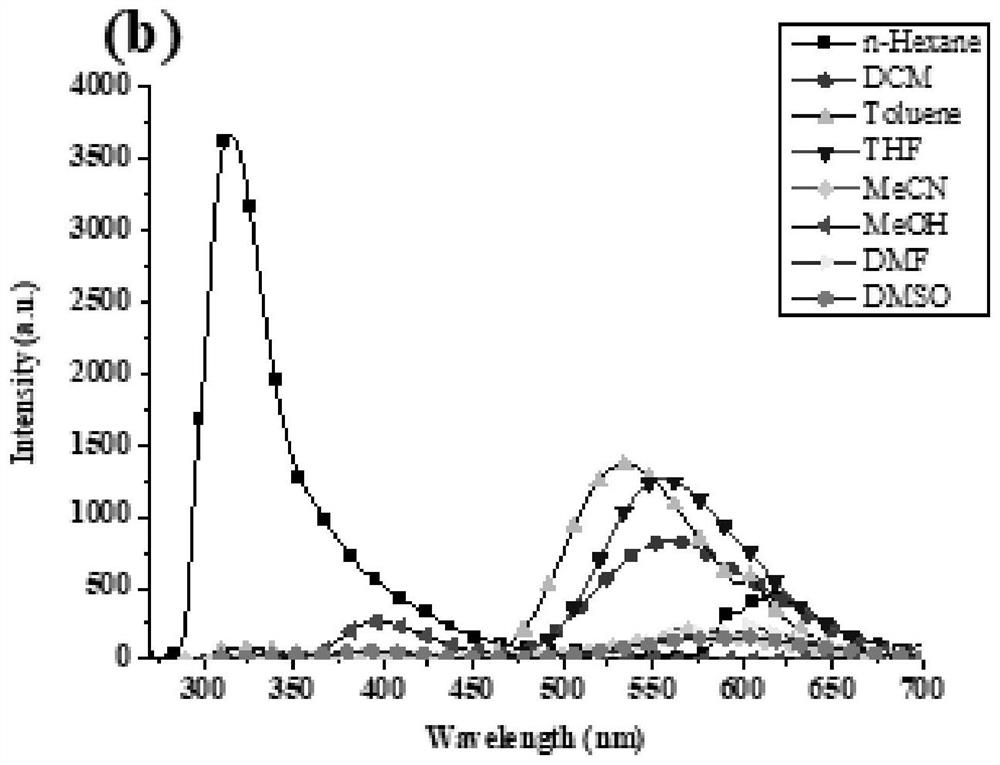
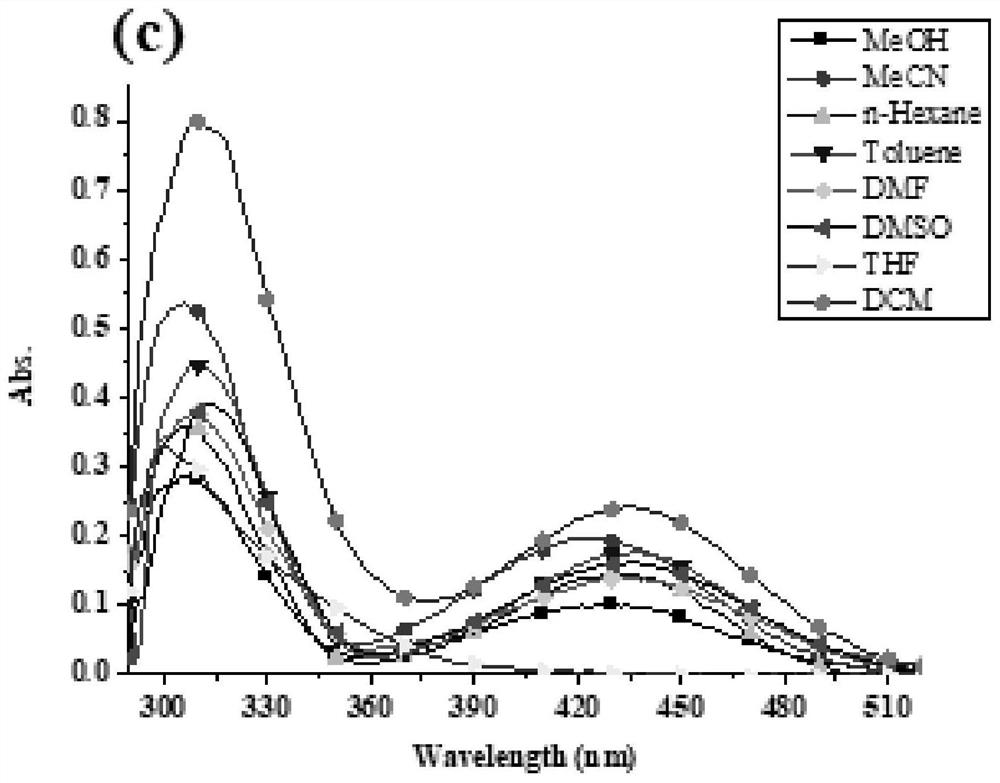
[0055] In this example, through a simple reaction in the synthesized benzothiadiazole- The aminopyrimidine or triphenylamine group was introduced on the base, and two benzothiadiazoles were synthesized- base derivatives, two benzothiadiazoles- The structural formula of the base derivative is shown in Table 1:
[0056] The structural formula of compound I and II of table 1
[0057]
[0058] Two benzothiadiazoles - The synthetic method of base derivative comprises the following steps:
[0059] Compound 1 reacts with 2-aminopyrimidine-5-boronic acid 2 to obtain compound I, and compound 1 reacts with 4-boronic acid triphenylamine 3 to obtain compound II. The specific steps are:
[0060] (1) Take compound 1 (2.0mmol), 2-aminopyrimidine-5-boronic acid 2 (4.8mmol), tetrakis(triphenylphosphine) palladium (0.06g) and potassium carbonate (0.53g) into a 100mL round bottom flask in turn 35mL of anhydrous toluene was added under the protection of argon, and the reaction was car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com