A hybridoma cell line secreting anti-bisamide compound monoclonal antibody and its application
A hybridoma cell line and monoclonal antibody technology, applied in the field of immunochemistry, can solve the problems of consumption, expensive equipment, high solvent, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
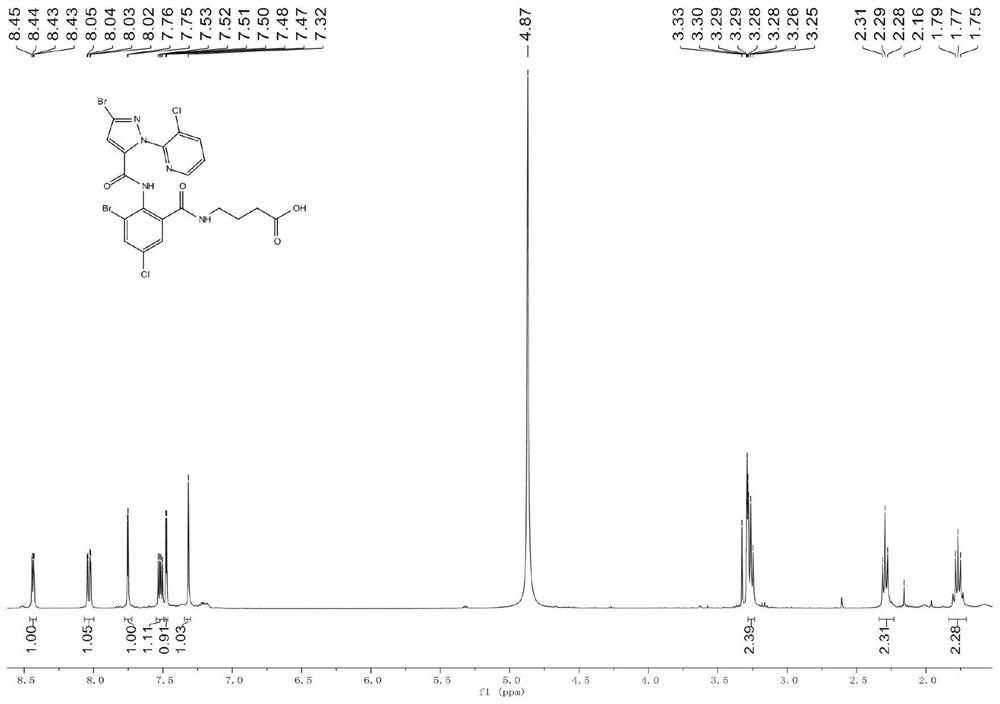
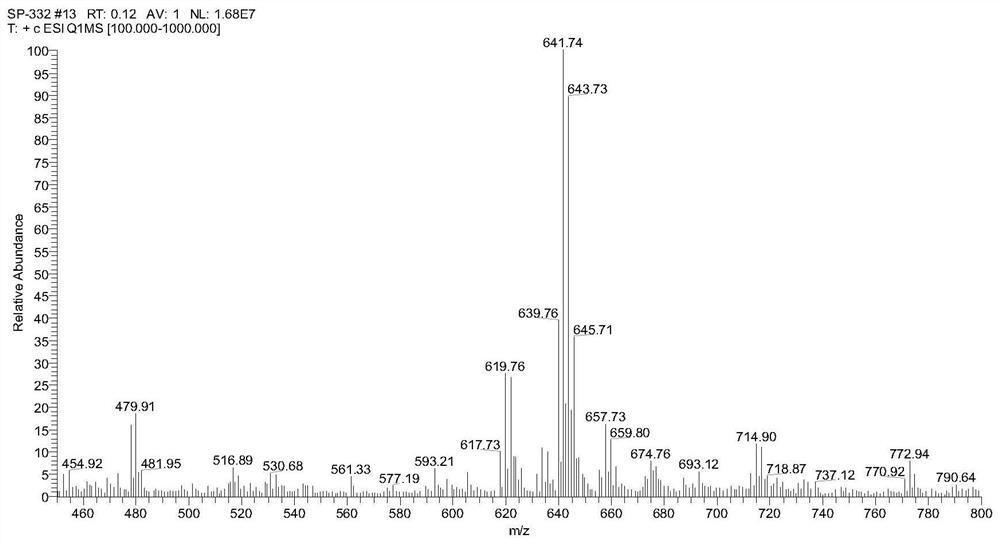
[0060] Example 1: Synthesis of Hapten (Hapten 1)
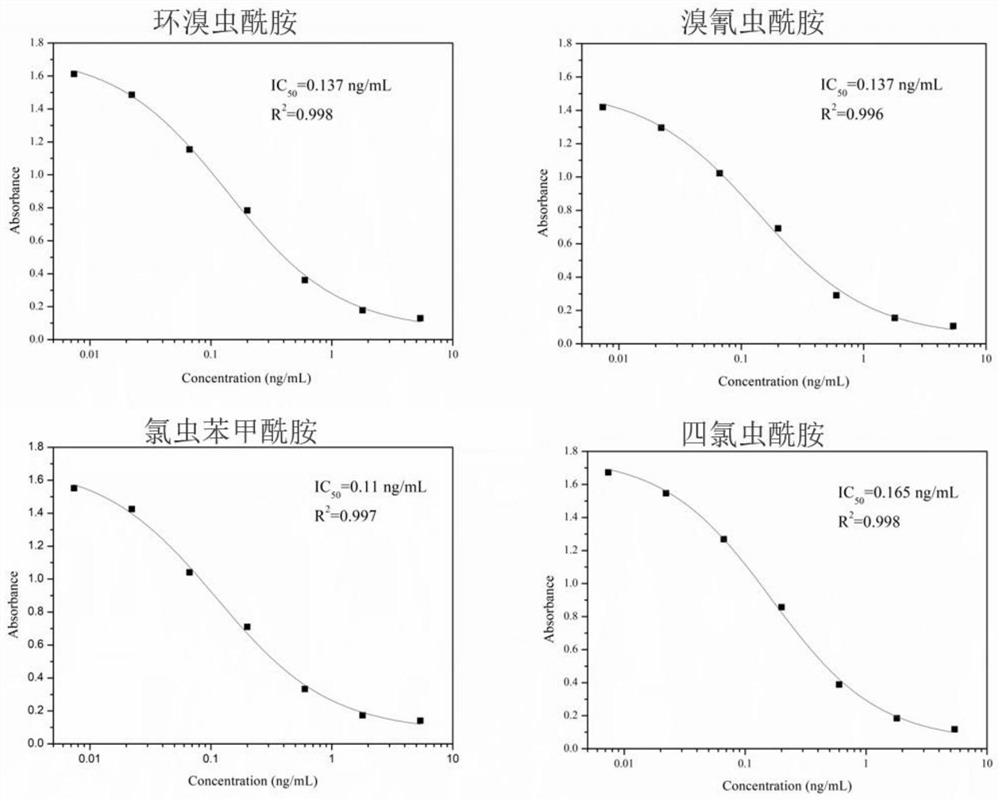
[0061] Since small molecules are not immunogenic and cannot stimulate mice to produce an immune response to produce antibodies, it is necessary to couple small molecules to proteins through protein linkage technology to obtain immunogenicity; commonly used in protein coupling techniques Active groups include amino, carboxyl, hydroxyl, mercapto, etc. In order to obtain monoclonal antibodies that can specifically recognize cyclobromide, cyantraniliprole, chlorantraniliprole, and tetrachlorantraniliprole, it is necessary to design and derive a For a good hapten, the specific derivatization steps are as follows.
[0062] Step 1: Synthesis of tert-butyl 4-(2-amino-3-bromo-5-chlorobenzamido)butyrate
[0063]
[0064] Add 1.0g (4.0mmol) 2-amino-3-bromo-5-chlorobenzoic acid, 10ml toluene and 0.80mL (12.0mmol) thionyl chloride to the reaction flask, reflux for 3 hours, remove the solvent, add 5mL tetrahydrofuran and wait Use; drop...
Embodiment 2
[0068] Example 2: Synthesis of Complete Antigen
[0069] Weigh 2.8mg hapten (Hapten 1), 1.6mg N-hydroxysuccinimide (NHS), dissolve in 300μL N,N-dimethylformamide (DMF), stir at room temperature for 10min; then weigh 2.6mg 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), fully dissolved with 100μL DMF, added to the hapten solution, stirred at room temperature for 6-8h (called Liquid A). Take 10mg of BSA, dilute it to 5mg / mL with 0.01M carbonate buffer (CBS) (referred to as solution B), then slowly add solution A to solution B drop by drop, react at room temperature overnight; then use 0.01M PBS solution Dialyzed to remove unreacted small molecular hapten to obtain the complete antigen, which was identified by UV absorption scanning method.
Embodiment 3
[0070] Embodiment 3: the synthesis of coating original
[0071]In order to improve the sensitivity, a new coating hapten (Hapten2) structure is designed as follows:
[0072]
[0073] The synthesis method of hapten (Hapten2) is similar to that of hapten (Hapten1). The specific steps for the synthesis of the coating agent are as follows: dissolve 3.6 mg hapten (Hapten2) and 2.3 mg N-hydroxysuccinimide (NHS) in 300 μL of anhydrous N,N-dimethylformamide (DMF), and stir the reaction at room temperature After 10 min, the hapten solution was obtained; 3.8 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) was dissolved in 100 μL of anhydrous DMF, and added to the hapten solution , stirred at room temperature and reacted for 6-8 hours to obtain liquid A; dilute 10 mg of chicken ovalbumin (OVA) with 1 mL of carbonate buffer solution (CBS) with a concentration of 0.01mol / L to obtain liquid B; Slowly add it into liquid B for reaction to obtain a reaction liquid;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com