Adrenaline sublingual spray for allergic shock as well as preparation method and application of adrenaline sublingual spray
An anaphylactic shock and epinephrine technology, applied in allergic diseases, aerosol delivery, medical preparations with inactive ingredients, etc. The effect of avoiding potential allergic reactions, simple preparation process, and reducing industrial costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] 1. Preparation of epinephrine sublingual spray
[0052] 1. Preparation of epinephrine sublingual sprays with different dosages of polysorbate 80
[0053] Accurately weigh 10 mg of epinephrine into an eggplant-shaped bottle, add 0.3 mL of 0.2M hydrochloric acid solution, and stir until the epinephrine is completely dissolved to obtain an epinephrine stock solution (Product 1). This process is completed in the dark.
[0054] Precisely weigh the absorption enhancer polysorbate 80 and the metal ion chelating agent ethylenediaminetetraacetic acid disodium (EDTA2Na) of the prescribed amount, add 0.7mL of pure water, so that the total volume of product 2 and product 1 in this step is the full amount of 1mL, Adjust temperature to 25°C, rotor speed 100r / min, stop heating after stirring for 2 hours, cool to room temperature naturally, add pH regulator citric acid and sodium citrate to adjust pH value to 3-4, and obtain excipient dilution (product 2).
[0055] Combine product 1 a...
Embodiment 21
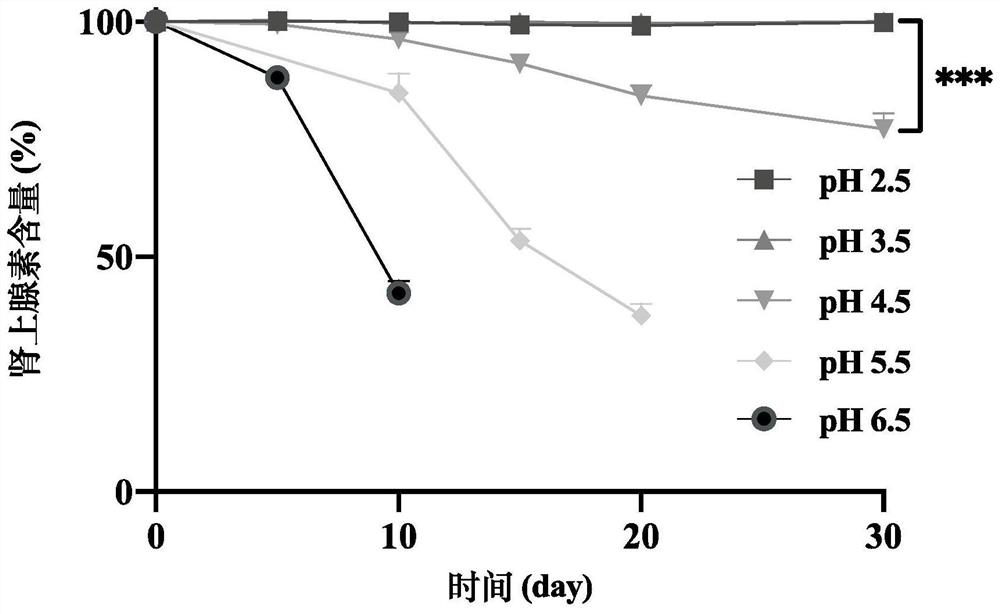
[0084] Embodiment 21 does not add the stability of the adrenaline target concentration solution (10mg / mL) of other auxiliary materials
[0085] Accurately weigh 10mg of epinephrine and add it to an eggplant-shaped bottle, add 0.3mL of 0.2M hydrochloric acid solution, stir until the epinephrine is completely dissolved, add 0.7mL of pure water until the total volume is 1mL, add pH regulators citric acid and sodium citrate respectively Adjust the final pH value to 2.5, 3.5, 4.5, 5.5, and 6.5 to obtain 10 mg / mL target concentration solutions of adrenaline with different pH values.
[0086] After placing the 10 mg / mL target concentration solutions of adrenaline with different pH values for one month, the content of adrenaline solutions with pH 4 and above significantly decreased, the color of the solutions changed significantly, and the impurity content increased significantly; the content of adrenaline solutions below pH 4 did not Significant changes occurred, and the solution h...
Embodiment 22
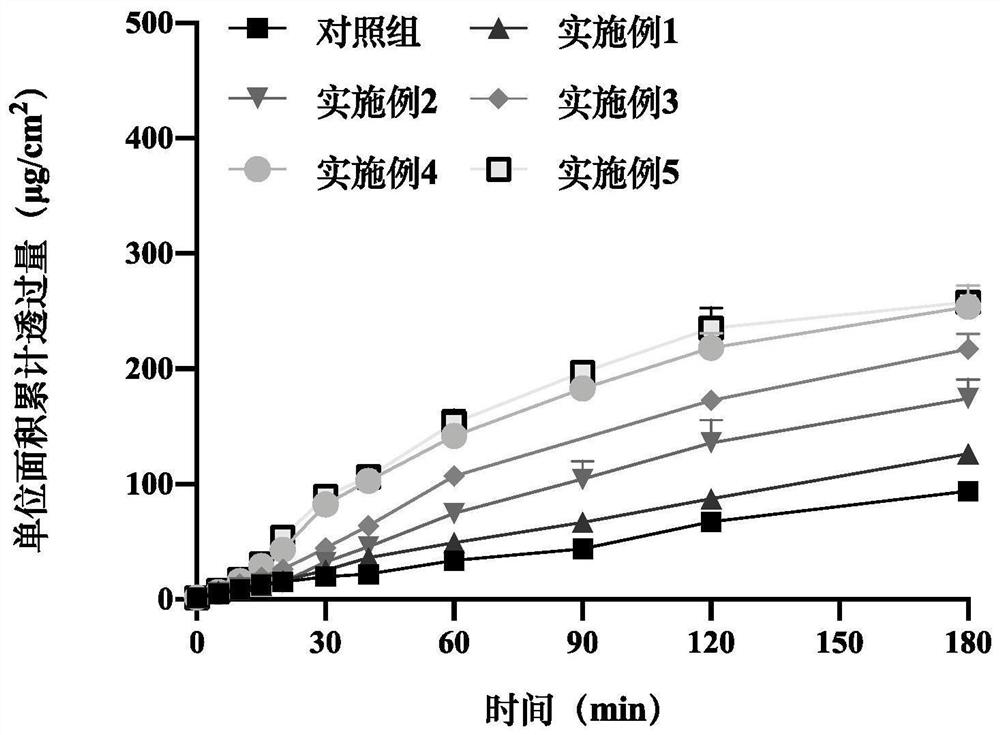
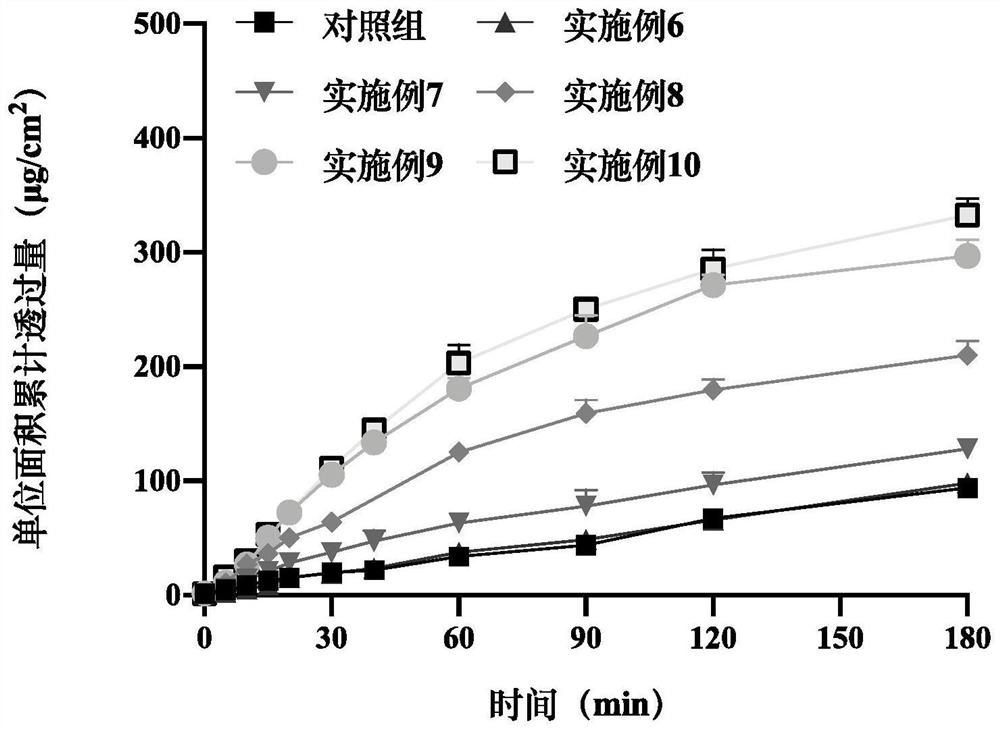
[0087] Sublingual mucosal permeability in vitro of embodiment 22 epinephrine sublingual spray
[0088] The oral mucosa of pigs was selected as the experimental model, and divided into a control group (blank adrenaline sublingual spray without absorption enhancer in Comparative Examples 1-4), and a preparation group (different prescriptions in Examples 1-20). The Franz diffusion cell was selected for in vitro permeation experiments. After administration, samples were taken at 0, 5, 10, 15, 20, 30, 40, 60, 90, 120, and 180 minutes, and the adrenaline content was detected by high performance liquid chromatography, and compared with the control Groups were compared to evaluate the in vitro mucosal permeability of adrenaline spray prepared by different prescriptions ( figure 2 , 3 , 4, 5, 6). The results are shown in the table below.
[0089] Table 5 Parameters of mucosal penetration of epinephrine within 30 minutes under different polysorbate 80 dosages (mean±SEM, n=3)
[009...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com