Preparation method of high-purity nateglinide capsule
A nateglinide and high-purity technology, applied in the field of preparation of high-purity nateglinide capsules, can solve the problems of unstable quality, low bioavailability, poor dissolution rate, etc. The effect of uniform dispersion and improved dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
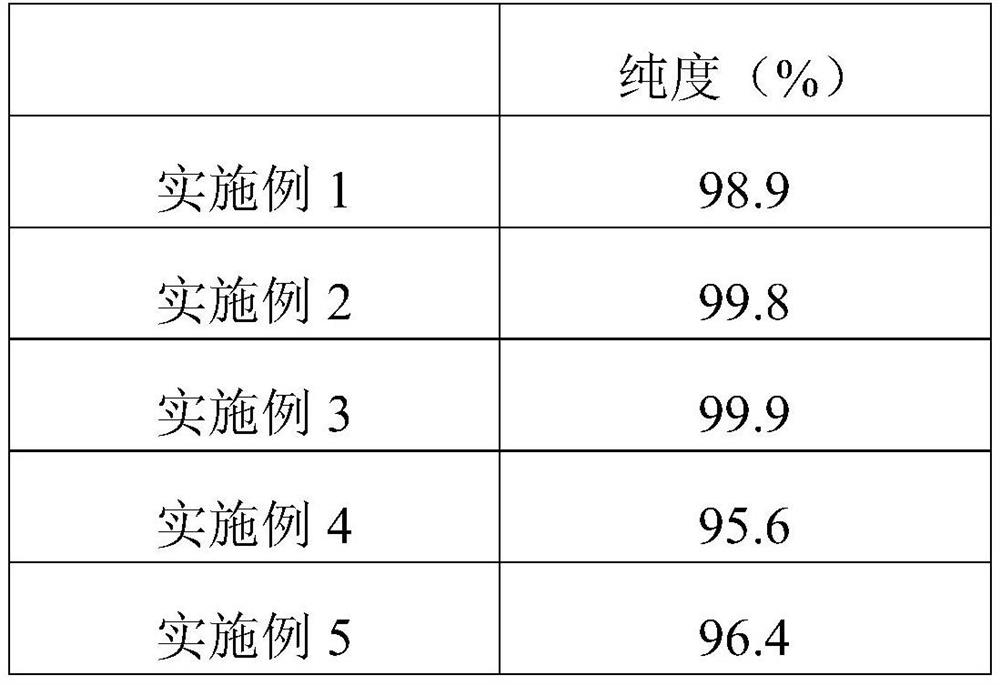
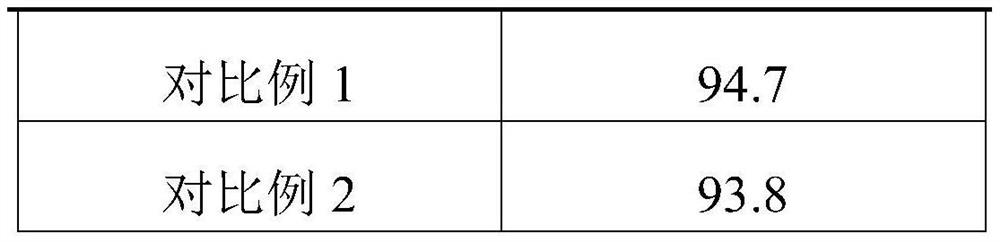
Embodiment 1
[0026] A preparation method of high-purity nateglinide capsules: comprising the following steps:
[0027] Preparation of S1 and H-type nateglinide: after condensing trans-4-isopropylcyclohexanecarbonyl chloride with D-phenylalanine, the crude crystal of nateglinide type B was obtained, and then at 15°C Perform low-temperature pulverization to obtain microparticle crystal seeds, add a crystallizing agent and then heat to 35 ° C to guide crystallization. The crystallizing agent is mixed with water-soluble phenolic and nicotinamide with a mass ratio of 0.3:1, and water with a mass-to-volume ratio of 2:1 is added to obtain crystals. agent to obtain white crystals of H-type nateglinide;
[0028] S2, the preparation of nanoemulsion: nanoemulsion comprises the following raw materials by weight: 13 parts of H-type nateglinide, 20 parts of triethanolamine, 10 parts of polysorbate 80, 3 parts of camellia oil, 2 parts of cyclohexyl propylene glycol, 2 parts of cross-linked carboxyl 1 pa...
Embodiment 2
[0032] A preparation method of high-purity nateglinide capsules: comprising the following steps:
[0033] Preparation of S1 and H-type nateglinide: after condensing trans-4-isopropylcyclohexanecarbonyl chloride with D-phenylalanine, a crude crystal of nateglinide type B is obtained, which is then pulverized at low temperature to obtain Microparticle seed crystals, add crystallization agent and then heat to 40 °C to guide crystallization to obtain H-type nateglinide white crystals. :1 water;
[0034] S2. Preparation of nanoemulsion: The nanoemulsion includes the following raw materials in parts by weight: 30 parts of H-type nateglinide, 25 parts of triethanolamine, 10 to 15 parts of polysorbate, 3 to 8 parts of castor oil, and 4 parts of polysorbate 400 , 1-3 parts of cross-linked polyvinylpyrrolidone, 1.5 parts of cationic polyacrylamide emulsion;
[0035] The preparation method of the nanoemulsion: dissolving the H-type nateglinide white crystal obtained by S1 in an alcohol...
Embodiment 3
[0038] A preparation method of high-purity nateglinide capsules: comprising the following steps:
[0039] Preparation of S1 and H-type nateglinide: after condensing trans-4-isopropylcyclohexanecarbonyl chloride with D-phenylalanine, the crude crystal of nateglinide type B was obtained, and then at 18°C Perform low-temperature pulverization to obtain microparticle seed crystals, add a crystallizing agent and then heat to 38 ° C to guide crystallization to obtain white crystals of H-type nateglinide. Water with a volume ratio of 3:1;
[0040] S2, the preparation of nanoemulsion: nanoemulsion comprises the following raw materials by weight: 18 parts of H-type nateglinide, 23 parts of n-propyl ester, 12 parts of polysorbate 60, 5 parts of olive oil, 3 parts of polyethylene glycol, 2 parts of copolyvidone, 1.0 part of acrylic acid;
[0041] The preparation method of the nanoemulsion: dissolving the H-type nateglinide white crystal obtained from S1 in an alcohol solvent, adding n-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com