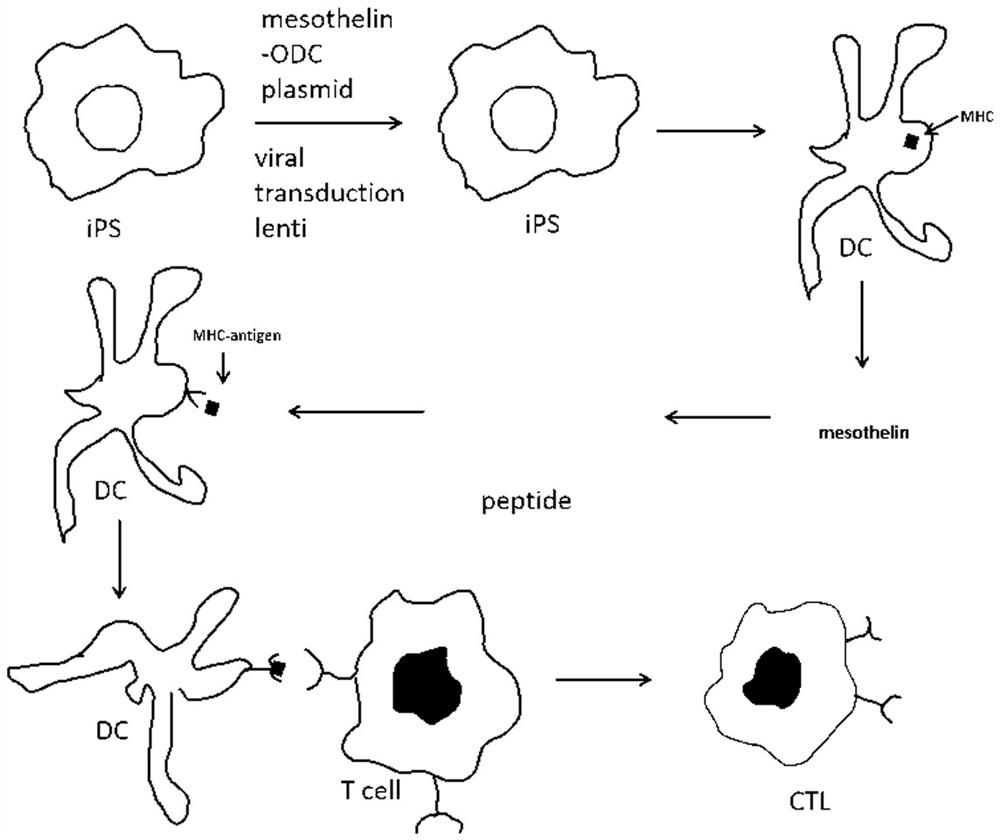
Mesothelin-ODC422-461 fusion protein expressed by DCs (dendritic cells) and application of mesothelin-ODC422-461 fusion protein
A fusion protein and cell technology, which is applied in the direction of fusion polypeptide, animal cells, cell culture active agents, etc., can solve the problems of immunity and antigenicity differences, achieve short half-life, improve antigen presentation efficiency, and have little toxicity and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
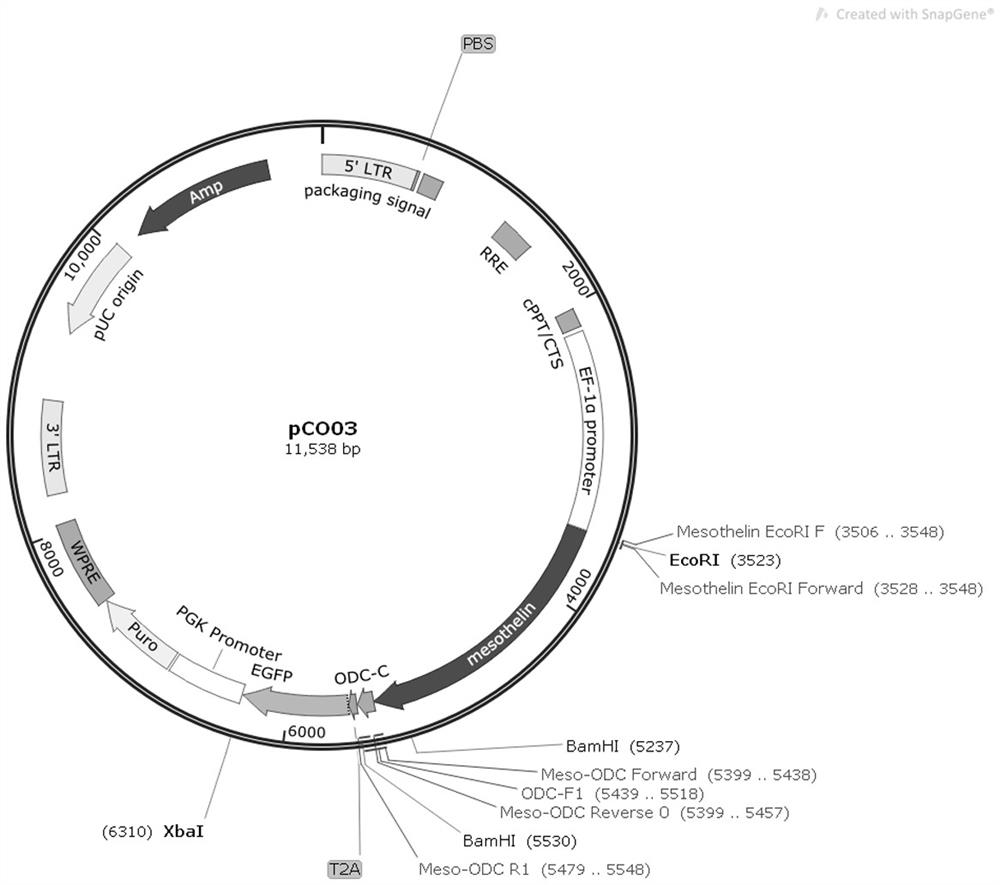
[0044] Example 1. The source of Mesothelin Use the primer pair Mesothelin EcoRI F (TTCAGGTGTCGTGATTCGAATTCATGGCCTTGCCAACGGCTCG) (SEQ ID NO.4) and Mesothelin BamHI R (AGGCTGCCTCTGCCCTCGGATCCTCAGGCCAGGGTGGAGGCTAGGA) (SEQ ID NO.5) to amplify the MSLN CDS sequence (SEQ ID NO.5) from human cNDA .6).
[0045] The PCR amplification conditions are:
[0046] I-5TM 2X High-Fidelity Master Mix: 12.5ul
[0047] 10uM Mesothelin EcoRIF: 0.75ul
[0048] 10uM Mesothelin BamHIR: 0.75ul
[0049] Human cDNA: 250ng
[0050] ddH2O: up to 25ul
[0051] The amplification program was: 98°C for 3 min; 98°C for 10 s, 56°C for 10 s, and 72°C for 10 s, a total of 30 cycles.
[0052] And purified and recovered; TOPO connected MSLN CDS sequence and pClone007 Versatile Simple Vector (Qingke Bio, cat.007VS), transformed into DH5α Escherichia coli competent, coated with ampicillin (Amp) resistant LB plate, picked single clone colony, Enzyme digestion and sequencing verification.
[0053] Pick 12 monocl...
Embodiment 2
[0055] The construction of embodiment 2.MSLN-ODC1425-461 eukaryotic expression vector:
[0056] PEST sequences rich in proline, glutamic acid, serine, and threonine are associated with rapid protein degradation. We used ePESTFind (http: / / www.bioinformatics.nl / cgi-bin / emboss / epestfind) to detect MSLN protein Analysis was performed and no potential PEST sequences were found. After the MSLN antigen is produced in the cell, although it can be degraded into small molecular peptides by the proteasome present in the cytoplasm, its half-life is longer. In order to reduce its half-life, we fused the ODC1425-461 sequence (SEQ ID NO.9) to the C-terminus of the MSLN protein to construct a vector pCO26, while pCO32 was used as a control without the ODC1425-461 sequence.
[0057] The experimental vector pCO26 was constructed using the vector pCO13 in our laboratory as a template, and the primer pair pCO26-F (CCATTTCAGGTGTCGTGATTCGAATTCGCCACCATGGCCTCCTCCG) (SEQ ID NO.10) and pCO26-R (GTCGAG...
Embodiment 3
[0075]Example 3. In vivo expression identification of MSLN fusion protein The vectors pCO26 and pCO32 constructed above were transfected into HEK293 T cells using the transfection reagent lipo2000, and the cells were visually detected 24 hours later. The experimental method is as follows:
[0076] Transfection: The day before the experiment, use the automatic cell counter Countstar-IC1000 and its supporting software CountStar BioTech to count the HEK293 cells, inoculate 2×105 cells / well in a 12-well plate, and culture the cells at 37°C and 5% CO2 Incubate overnight in the box. 30 minutes before transfection, replace the medium with fresh DMEM medium containing 5% FBS, 1 mL per well. For transfection, take 1.6 μg of the corresponding plasmid from each transfection well, add it to 300 μL of FBS-free reduced serum DMEM medium, mix well, add 4.0 μL of transfection reagent, mix gently, and place at room temperature for 18 minutes. Gently drop the mixture of plasmid and transfecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com