Method for preparing artemisinin by adopting continuous flow pipeline reaction
A technology of artemisinin and reactor, which is applied in the field of pharmaceutical industry to achieve the effect of shortening the reaction time, reducing the amount of solvent, and reducing the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 artemisinin preparation
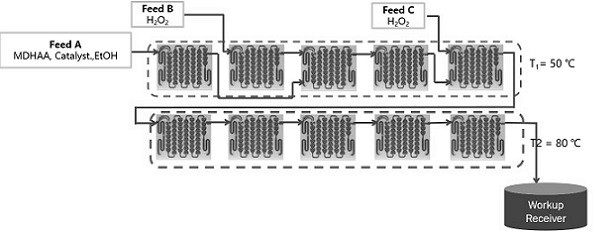
[0029] Step 1) reaction, using a continuous flow reactor to prepare MPDHAA (see figure 1 Schematic diagram of the continuous flow reactor process)
[0030] Reaction materials: starting material MDHAA, lanthanide catalyst, sodium molybdate, inorganic alkali sodium hydroxide, water, organic solvent absolute ethanol, hydrogen peroxide.
[0031] The operation process is as follows:
[0032] 1. Material configuration: Weigh 2730g of absolute ethanol, 390g of lanthanum nitrate hexahydrate and 220g of sodium molybdate dihydrate into a 5L beaker, add 1122g of 2.5N sodium hydroxide aqueous solution under stirring, and weigh 3000g of MDHAA and 9120.0g absolute ethanol is added in the container and mixed with the catalyst suspension, then placed in a shearing machine to shear,
[0033] 2. Preparation of 50% hydrogen peroxide: put 50% hydrogen peroxide in a 1L narrow-mouth bottle and put it in an ice water bath for later use.
[0034] 3. C...
Embodiment 2
[0049] Example 2 The selection of the proportion of hydrogen peroxide and moisture added to the continuous flow microchannel in step 1) of the reaction
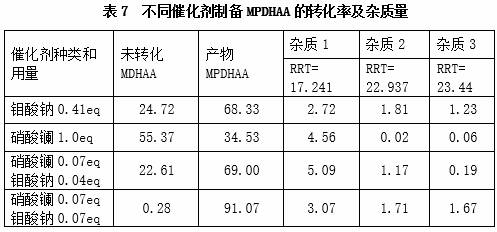
[0050] Taking 15eq of hydrogen peroxide as an example, the effect of different distribution ratios of two parts of hydrogen peroxide on the product conversion rate and quality is studied. The experimental method refers to the step 1) reaction operation process of Example 1, and the distribution ratio of hydrogen peroxide in the first module and the fifth module (two parts) is respectively set to 1:4.9, 1:1, 4.9:1 in three different ratios for continuous The flow reaction was carried out, and the content of MPDHAA in the product was determined by HPLC. The results are shown in Table 1 below.
[0051]
[0052] The results in Table 1 show that in the case of a sufficient amount of hydrogen peroxide, the ratio of the two parts of hydrogen peroxide is different, which has little effect on the conversion rate and quality, but t...
Embodiment 3
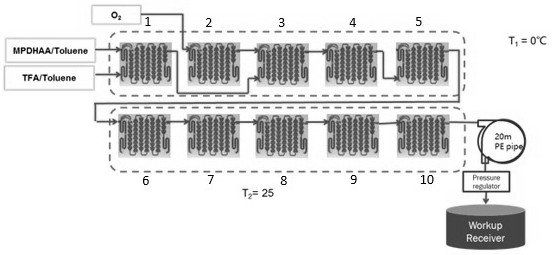
[0053] Example 3 2) The influence of the concentration of oxygen in the step on the reaction
[0054] Referring to step 2) of the reaction process operation in Example 1, under the condition that other conditions remain unchanged, the influence of the oxygen concentration (oxygen dosage) on the reaction is investigated. The results are shown in Table 2.
[0055]
[0056] The results in Table 2 show that increasing O 2 Flux has little effect on the conversion rate of artemisinin (QHS). Therefore, the amount of oxygen (molar ratio to MPDHAA) can be in the range of 2.3-3.0eq. Based on cost saving and safety considerations, the amount of 2.32eq can be preferred.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com