High-conductivity sulfur-based positive electrode material for secondary battery and secondary battery
A secondary battery, high conductivity technology, applied in the direction of secondary batteries, battery electrodes, positive electrodes, etc., can solve the problem of low cycle stability and rate performance, which affects the use of secondary batteries, and the conductivity of S@pPAN positive electrode materials Low-level problems, to achieve the effects of cycle stability and rate performance improvement, strong practicability, and high crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
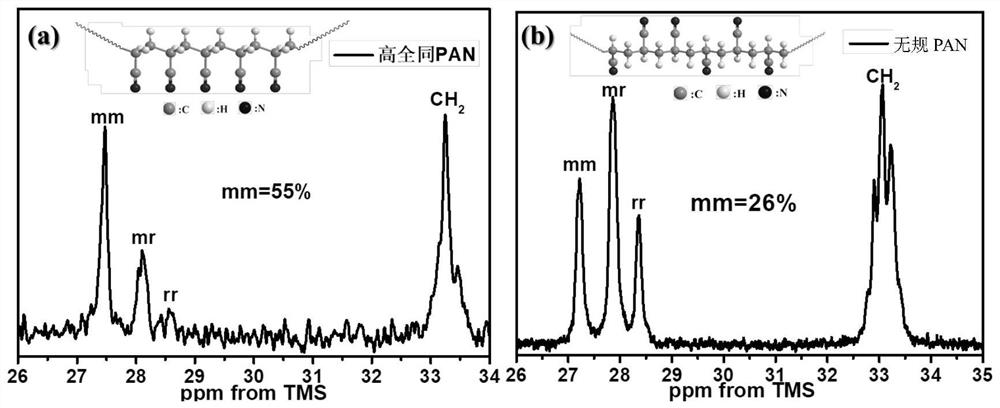
[0055] 30g of anhydrous CoCl 2 It was put into a three-necked flask, and after cooling in ice water for 30 min, the flask was filled with argon. Then 7g of acrylonitrile monomer and 0.15g of AIBN initiator were added, and the polymerization reaction was started at 60°C under magnetic stirring for 4h. After 6h of reaction, alternately washed with methanol and water, and then the obtained white powder was dried in a vacuum oven. After 24h, high isotactic polyacrylonitrile (the isotactic ratio of 55%) was obtained.
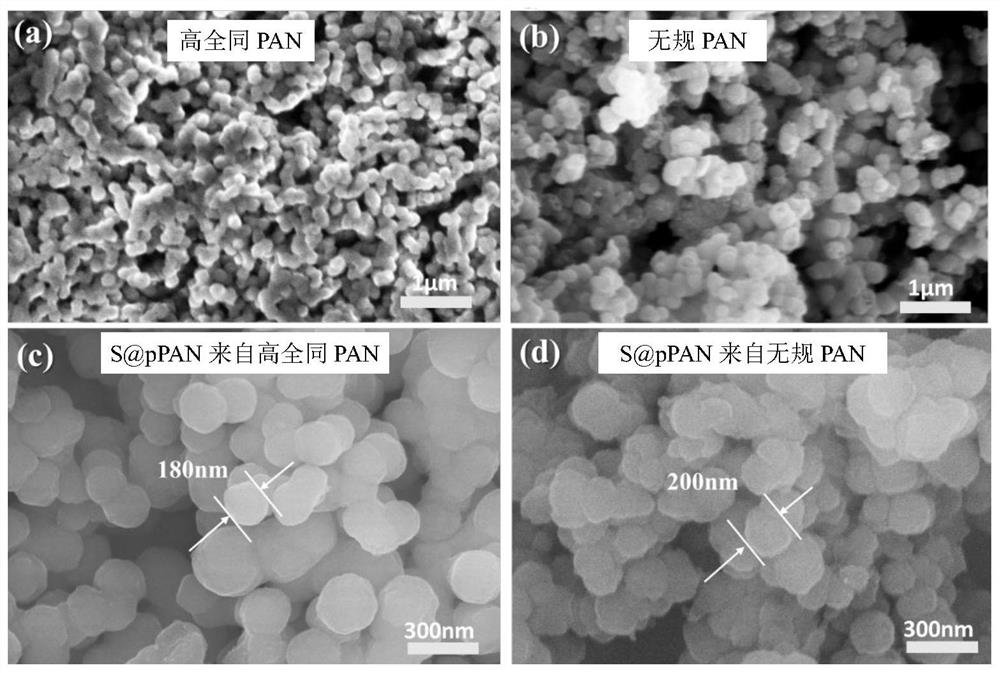
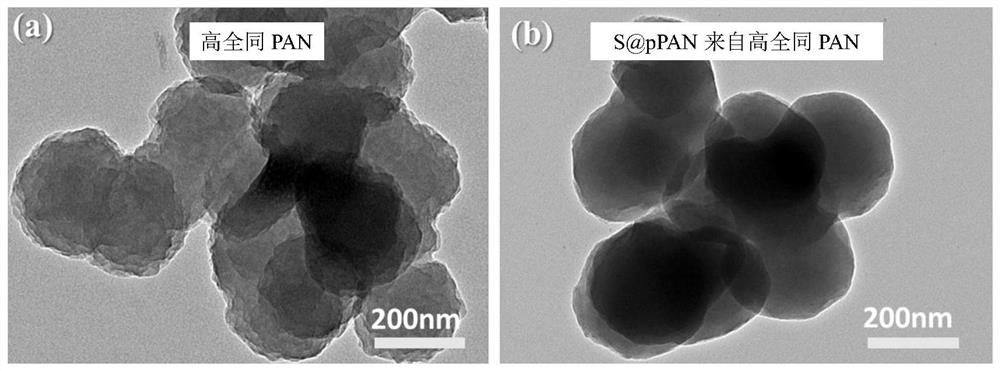
[0056] figure 1 and 2 The SEM and TEM images of the high isotactic polyacrylonitrile and the positive electrode material prepared in this example and the 1 random polyacrylonitrile and sulfur-based positive electrode material S@pPAN in the comparative example, it can be found that the particles of high isotactic polyacrylonitrile The diameter distribution is more uniform, the sphericity is better, and the surface is smoother.
[0057] like image 3 As shown, the...
Embodiment 2
[0061] 30g of anhydrous CoCl 2 It was put into a three-necked flask, and after cooling in ice water for 30 min, the flask was filled with argon. Then 7g of acrylonitrile monomer and 0.15g of AIBN initiator were added, and the polymerization reaction was started at 70°C under magnetic stirring for 4h. After 6h of reaction, alternately washed with methanol and water, and then the obtained white powder was dried in a vacuum oven. After 24h, high isotactic polyacrylonitrile (the isotactic ratio of 55%) was obtained.
[0062] High isotactic polyacrylonitrile, the isotonic ratio is much higher than that of random polyacrylonitrile, and the morphology is more regular, so its crystallinity is also much higher than that of random polyacrylonitrile, which is the corresponding group ( -AN) and the characteristic peaks of the material are shifted, such as Figure 4-5 shown.
[0063] Image 6 According to the DSC spectrogram analysis results of the high isotactic polyacrylonitrile prep...
Embodiment 3
[0067] 30g of anhydrous CoCl 2 It was put into a three-necked flask, and after cooling in ice water for 30 min, the flask was filled with argon. Then 7g acrylonitrile monomer and 0.15g AIBN initiator were added, and the polymerization reaction was started at 70°C under magnetic stirring for 4h. After 6h of reaction, the white powder was washed alternately with methanol and water, and then the obtained white powder was dried in a vacuum oven. After 24h, high isotactic polyacrylonitrile (the isotactic ratio of 55%) was obtained.
[0068] 2 g of the obtained high isotactic polyacrylonitrile and 16 g of elemental sulfur were added to ethanol for ball milling for 3 h, and after drying, the obtained powder was heated in a tube furnace at 350 °C for 5 h under a nitrogen atmosphere to obtain sulfur-based cathode material S@pPAN , the sulfur content in the material is 40.20wt%, and the electronic conductivity of the material is 6.7×10 -5 S / cm.
[0069] The battery assembly and test ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com