Nanogold confinement copper-based core-shell structure catalyst as well as preparation method and application thereof
A core-shell structure and nano-gold technology, applied in the preparation of hydroxyl compounds, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of easy loss of active components and poor catalyst stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
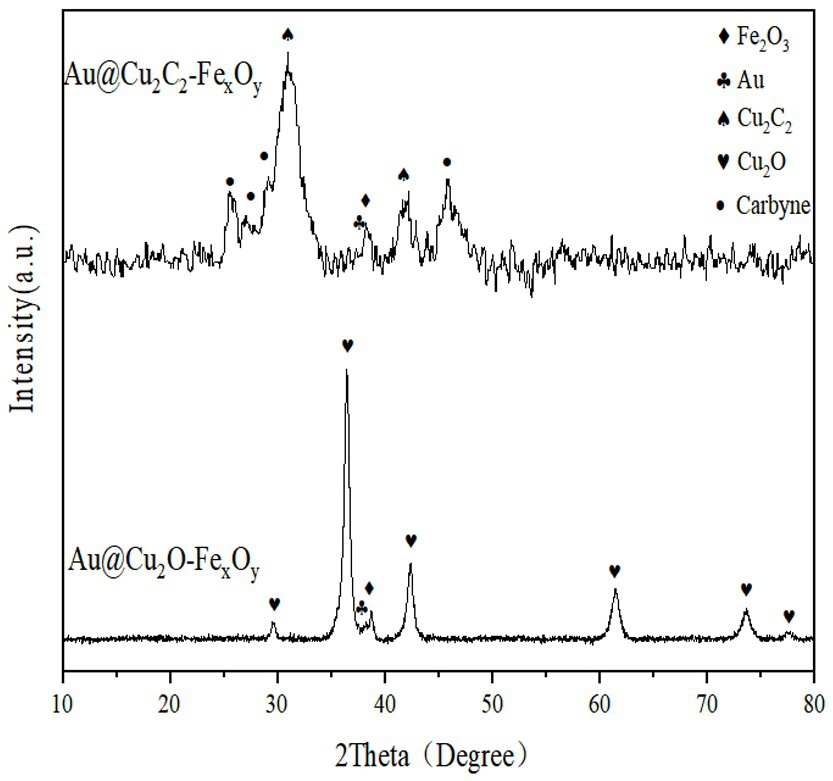
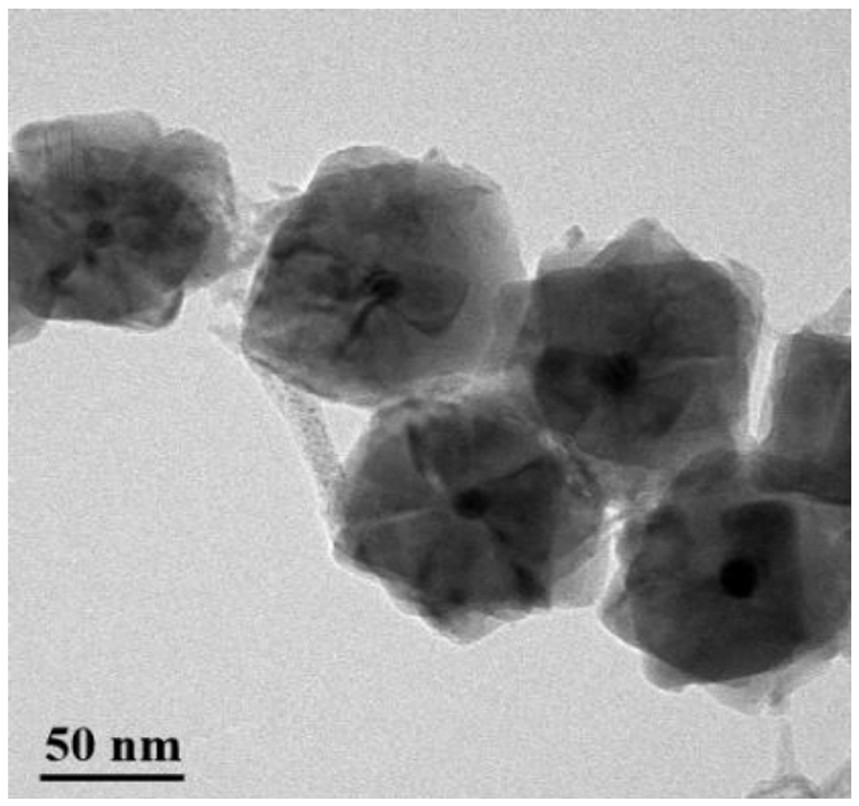
Image
Examples
Embodiment 1
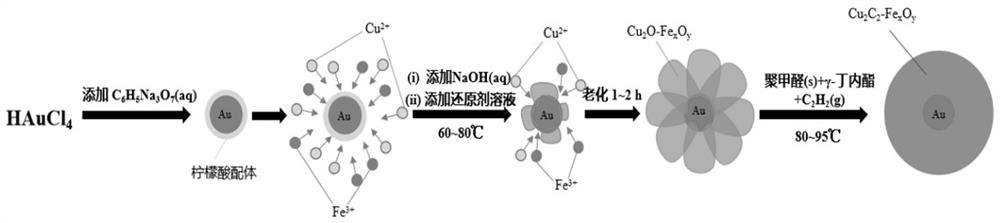
[0034] Weigh 0.05g of chloroauric acid and dissolve it in water to prepare 500 mL of chloroauric acid aqueous solution, and the mass concentration of chloroauric acid is controlled at 0.1 g / L; weigh 0.05 g of sodium citrate and dissolve it in water to prepare 10 mL of sodium citrate aqueous solution, the mass concentration of sodium citrate was controlled at 5 g / L; the prepared 500 mL chloroauric acid aqueous solution was heated and boiled in a microwave oven, and 4 mL sodium citrate aqueous solution was quickly added, and kept boiling for 10 min, until it dropped to After room temperature, the gold sol solution was obtained.
[0035] Weigh 18.2 g copper nitrate trihydrate, 10.85 g ferric nitrate nonahydrate, 6.06 g sodium lauryl sulfate and 3.03 g PVP, dissolve them in distilled water together, and control the mass concentration of 1 L copper at 4.79 g / L. The mass concentration of iron is controlled at 1.5 g / L, the mass concentration of sodium lauryl sulfate is controlled at ...
Embodiment 2
[0038] Weigh 0.06 g of chloroauric acid and dissolve it in water to prepare 300 mL of chloroauric acid aqueous solution. The mass concentration of chloroauric acid is controlled at 0.2 g / L; weigh 0.08 g of sodium citrate and dissolve it in water to prepare 10 mL of sodium citrate Aqueous solution, the mass concentration of sodium citrate was controlled at 8 g / L; 300 mL of chloroauric acid aqueous solution was heated and boiled in a microwave oven, and 3.3 mL of sodium citrate aqueous solution was added quickly, and kept boiling for 20 min. After room temperature, the gold sol solution was obtained.
[0039] Weigh 23.7 g copper nitrate trihydrate, 3.62 g ferric nitrate nonahydrate, 7.89 g sodium lauryl sulfate and 5.26 g PVP, dissolve them in distilled water together, and control the mass concentration of 1 L copper at 6.23 g / L. The mass concentration of iron is controlled at 0.5 g / L, the mass concentration of sodium lauryl sulfate is controlled at 7.89 g / L, and the mass concen...
Embodiment 3
[0042] Weigh 0.015 g of chloroauric acid and dissolve it in water to prepare 150 mL of chloroauric acid aqueous solution. The mass concentration of chloroauric acid is controlled at 0.1 g / L; weigh 0.10 g of sodium citrate and dissolve it in water to prepare 10 mL of sodium citrate aqueous solution, the mass concentration of sodium citrate was controlled at 10 g / L; heat and boil the prepared 150 mL chloroauric acid aqueous solution in a microwave oven, and quickly add 2.7 mL sodium citrate aqueous solution, keep boiling for 30 min, and wait until it drops to After room temperature, the gold sol solution was obtained.
[0043] Weigh 27.9 g copper nitrate trihydrate, 2.17 g ferric nitrate nonahydrate, 9.30 g sodium lauryl sulfate and 2.33 g PVP, dissolve them in distilled water together, and control the mass concentration of 1 L copper at 7.33 g / L. The mass concentration of iron is controlled at 0.3 g / L, the mass concentration of sodium lauryl sulfate is controlled at 9.30 g / L, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Shell thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com