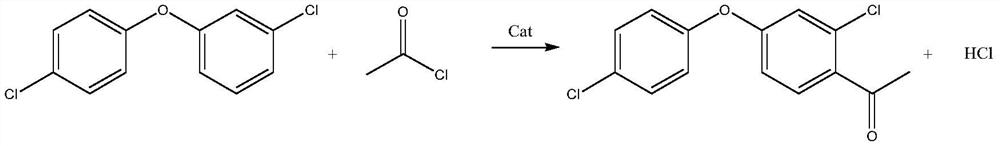
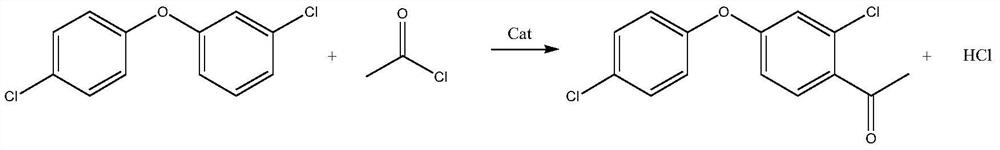
Synthesis method of 2-chloro-4-(4-chlorophenoxy)-acetophenone
A technology of chlorophenoxy and synthetic method, which is applied in the field of synthesis of 2-chloro-4-acetophenone, can solve the problems of complex operation, low recovery rate of solvent, low recovery rate of diphenyl ether ketone, etc., and achieve product The effect of high yield, simplified production process, and easy industrial implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
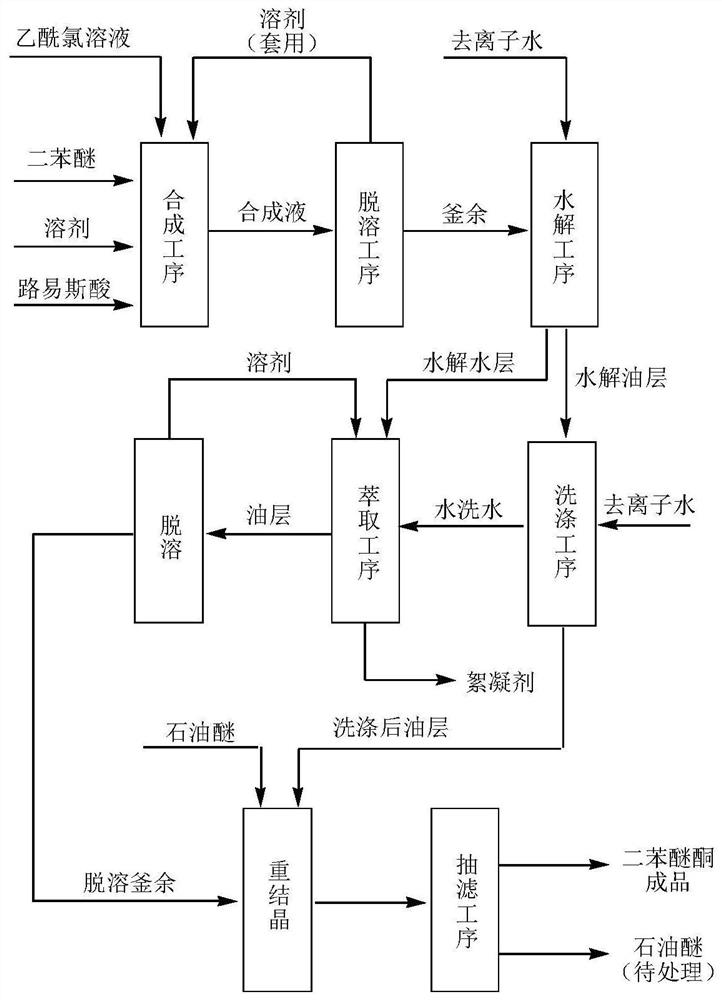
[0026] (1) Put 75.0 g of diphenyl ether (98.5%) into a 1000 ml four-necked flask, add 90.2 g of solvent dichloroethane, 49.2 g of catalyst aluminum trichloride, and cool down to 5°C. Prepare a uniform solution of 30.0g acetyl chloride and 90.0g dichloroethane, inject it into the reaction system with a metering pump, control the reaction dripping time for 2 hours, and the holding time for 4 hours. After the heat preservation is completed, a small amount of the reaction solution is hydrolyzed to obtain an oil layer, and the normalized gas spectrum detection shows that the content of diphenyl ether is 0.005%, and the content of diphenyl ether ketone is 95.2%. The synthesis device was changed to a distillation device, and 171.3 g of dichloroethane (95% fraction) was removed under negative pressure conditions, and the residue in the still was 132.7 g of black solid particles.
[0027] (2) Prepare a 1000mL four-neck flask, add 380g of deionized water, 20g of reagent hydrochloric aci...
experiment example 2
[0032] (1) Put 75.0 g (0.3 mol) of diphenyl ether (98.5%) into a 1000 ml four-neck flask, add 132 g (0.9 mol) of o-dichlorobenzene as a solvent, and 49.2 g of aluminum trichloride as a catalyst, and cool down to 5°C. Prepare 30.0g of acetyl chloride and 143g of o-dichlorobenzene to form a uniform solution, use a metering pump to inject into the reaction system, control the reaction dripping time for 2 hours, and the holding time for 4 hours. After the heat preservation is completed, a small amount of the reaction solution is hydrolyzed to obtain an oil layer, and the normalized gas spectrum detection shows that the content of diphenyl ether is 0.003%, and the content of diphenyl ether ketone is 95.9%. The synthesis device was changed to a distillation device, and 264g of o-dichlorobenzene (96% fraction) was removed under negative pressure, and the residue in the still was 136.3g of black solid particles.
[0033] (2) Add 380g of deionized water and 20g of reagent hydrochloric ...
experiment example 3
[0038] (1) Put 75.0 g (0.3 mol) of diphenyl ether (98.5%) into a 1000 ml four-neck flask, add 83.2 g (0.90 mol) of solvent toluene, and 89.9 g of catalyst tin chloride, and cool down to 5°C. Prepare 30.0g acetyl chloride and 82.7g toluene into a homogeneous solution, use a metering pump to inject into the reaction system, control the reaction dripping time for 2 hours, and the holding time for 4 hours. After the heat preservation is completed, a small amount of the reaction solution is hydrolyzed to obtain an oil layer, and the normalized gas spectrum detection shows that the content of diphenyl ether is 0.008%, and the content of diphenyl ether ketone is 94.9%. The synthesis device was changed to a distillation device, and 157.6 g of toluene (95% cut) was removed under negative pressure conditions, and the residue in the still was 174.3 g of black solid particles.
[0039] (2) Add 380g of deionized water and 20g of reagent hydrochloric acid into a 1000mL four-neck flask, cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com