Ferrocene-triazole Schiff base derivative and preparation method thereof
A technology for ferrocene triazole Schiff bases and derivatives, which is applied in the field of ferrocene derivatives and their preparation, can solve the problems of poor electron mobility of ferrocene and its derivatives, and achieve high degree of binding firmness, Prevents loss and prolongs service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
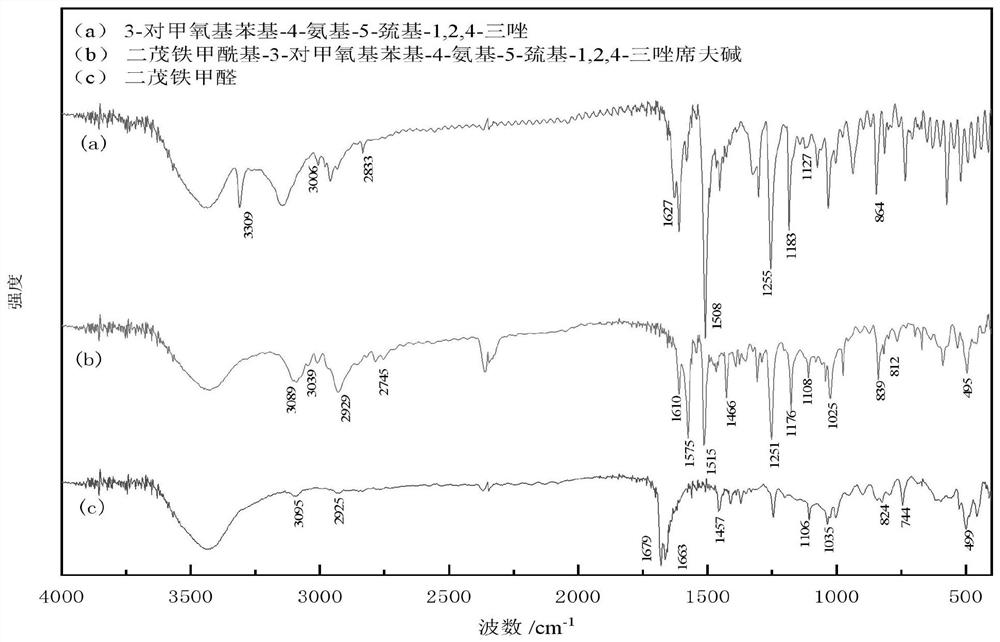
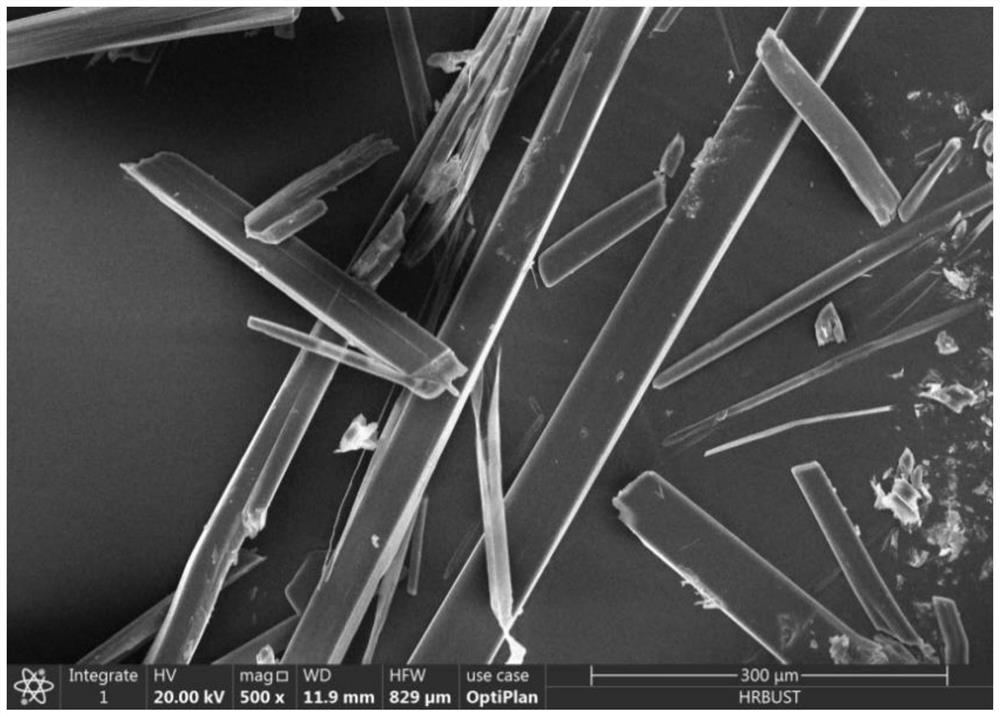
Image
Examples
specific Embodiment approach 1
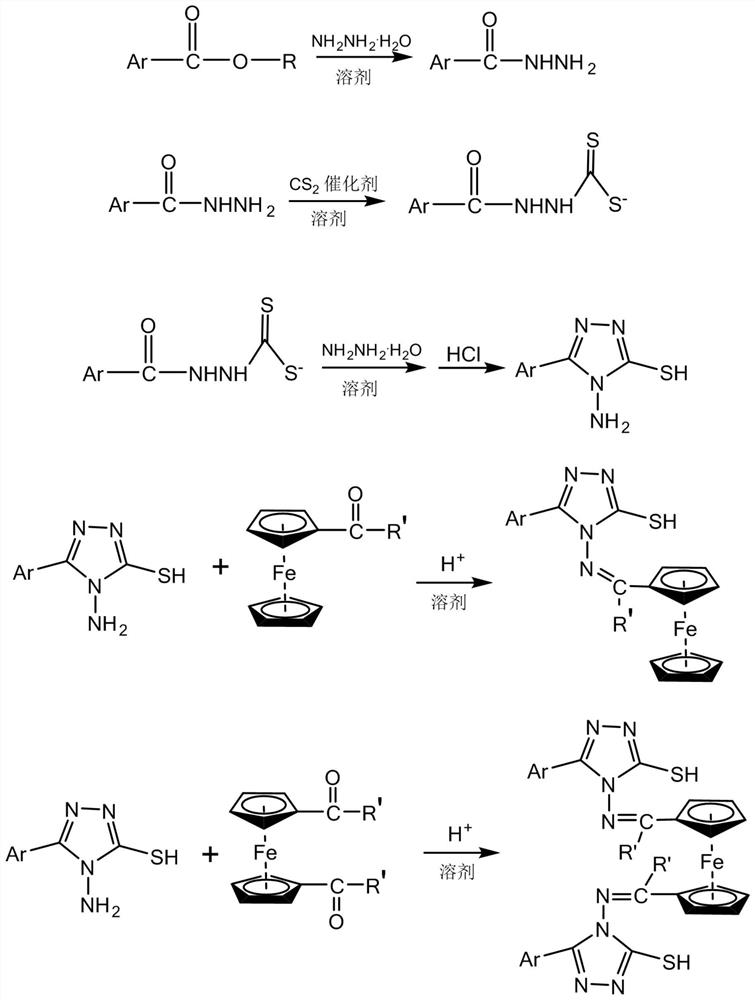
[0019] Embodiment 1: This embodiment is a ferrocene triazole Schiff base derivative, and its general structural formula is: Ar in the general structural formula is
[0020]
[0021] R' is -H, -CH 3 or -CH 2 CH 2 CH 3 .
specific Embodiment approach 2
[0022] Specific embodiment two: This embodiment is the preparation method of ferrocene triazole Schiff base derivative, and is specifically completed according to the following steps:
[0023] 1. Preparation of aryl formate hydrazide: add aryl formate into solvent I, stir until the aryl formate is completely dissolved, and then dropwise into hydrazine hydrate at a rate of 1 mL / min to 2 mL / min under stirring conditions, Hydrazine hydrate is completely added dropwise and heated to reflux temperature, then refluxed for 3h~10h, cooled to room temperature, and then left to stand until no crystals are precipitated, suction filtration to obtain filter cake I, and filter cake I is washed with distilled water for 2~5 times, the washed filter cake I was obtained, and the washed filter cake I was vacuum-dried for 3 h to 8 h at a temperature of 60° C. to obtain an arylformyl hydrazide;
[0024] 2. Preparation of arylformyl hydrazide dithioformate: add the catalyst into solvent II, stir un...
specific Embodiment approach 3
[0032] Embodiment 3: The difference between this embodiment and Embodiment 2 is: the volume ratio of the amount of aryl formate described in step 1 to the volume of solvent I is 1mol:(50-500) mL; step 1 The mol ratio of aryl formate and hydrazine hydrate described in the middle is 1:(1~10); In step 1, the quality of filter cake 1 and the volume ratio of distilled water are 1g:(30~ 50) mL. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com