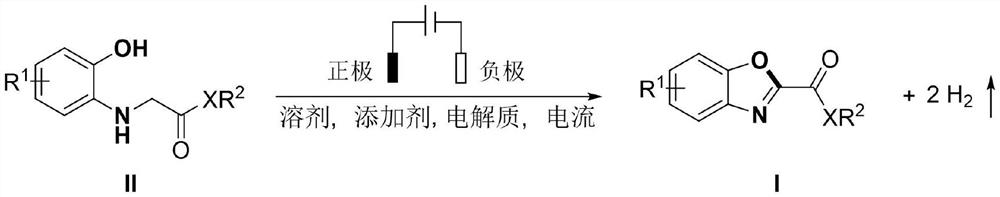
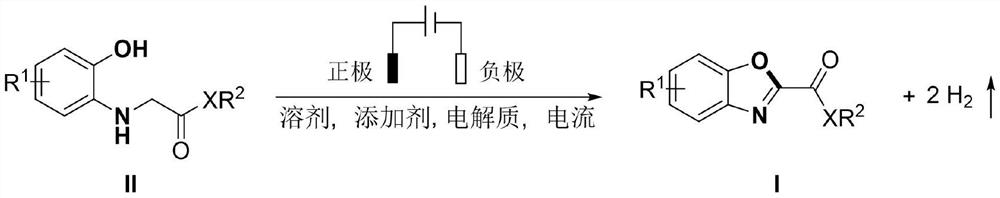
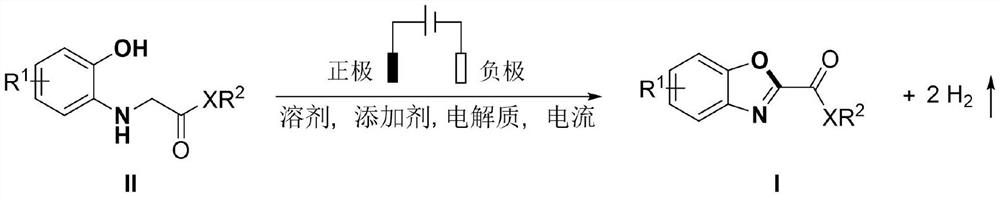
Electrochemical synthesis method of 2-substituted benzoxazole derivative
A technology of benzoxazole and synthesis method, applied in the field of electrochemical synthesis of 2-substituted benzoxazole derivatives, can solve the problems of large amount of three wastes, harsh reaction conditions, etc. Implementation value and socio-economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of ethyl 2-benzoxazole carboxylate (Ia)
[0032] N-(2-Hydroxyphenyl)-glycine ethyl ester (59 mg, 0.3 mmol), acetic acid (18 mg, 0.3 mmol), tetrabutylammonium tetrafluoroborate (50 mg, 0.15 mmol) and acetonitrile (6 mL) were added to the reaction into the tube, and insert a mesh glassy carbon electrode and a platinum electrode, adjust the current to a constant current of 8mA, and react at 40°C under nitrogen protection for 24 hours, and obtain orange-yellow solid 2-benzoxazole ethyl carboxylate through silica gel column chromatography Ester (Ia) 43 mg, yield: 74%, melting point 88-91.6 ° C, the structural formula of Ia is:
[0033]
[0034] 1 H NMR (400MHz, CDCl 3 )δ7.89(d, J=8.0Hz, 1H), 7.66(d, J=8.0Hz, 1H), 7.52(t, J=7.6Hz, 1H), 7.45(t, J=7.6Hz, 1H) ,4.55(q,J=7.2Hz,2H),1.49(t,J=7.2Hz,3H); 13 CNMR (100MHz, CDCl 3 )δ156.1, 152.6, 150.7, 140.4, 128.3, 125.4, 122.5, 111.8, 63.6, 14.4; HRMS (ESI): calcd for C 10 h 9 NO 3 (M+H) + :19...
Embodiment 2
[0035] Embodiment 2: the preparation of ethyl 2-benzoxazole carboxylate (Ia)
[0036] N-(2-hydroxyphenyl)-glycine ethyl ester (59mg, 0.3mmol), p-toluenesulfonic acid (52mg, 0.3mmol), tetrabutylammonium tetrafluoroborate (50mg, 0.15mmol) and acetonitrile (6mL) Add it into the reaction tube, and insert the mesh glassy carbon electrode and platinum electrode, adjust the current to a constant current of 8mA, and react at 40°C under nitrogen protection for 24 hours, and separate by silica gel column chromatography to obtain orange-yellow solid 2-benzoxane Ethyl azole carboxylate (Ia) 11 mg, yield: 20%.
Embodiment 3
[0037] Embodiment 3: the preparation of ethyl 2-benzoxazole carboxylate (Ia)
[0038] N-(2-Hydroxyphenyl)-glycine ethyl ester (59 mg, 0.3 mmol), formic acid (14 mg, 0.3 mmol), tetrabutylammonium tetrafluoroborate (50 mg, 0.15 mmol) and acetonitrile (6 mL) were added to the reaction into the tube, and insert a mesh glassy carbon electrode and a platinum electrode, adjust the current to a constant current of 8mA, and react at 40°C under nitrogen protection for 24 hours, and obtain orange-yellow solid 2-benzoxazole ethyl carboxylate through silica gel column chromatography Ester (Ia) 31 mg, yield: 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com