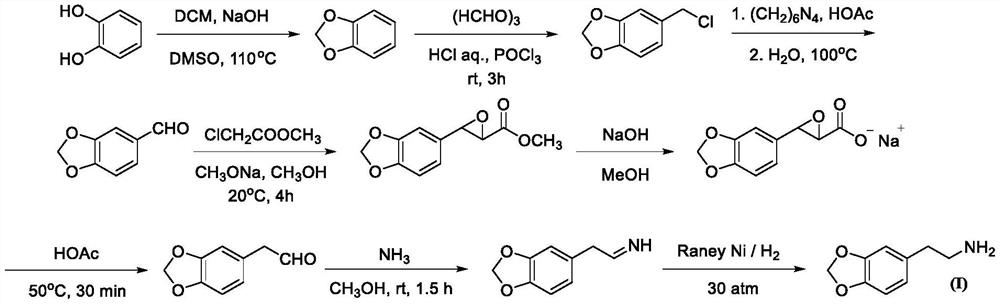
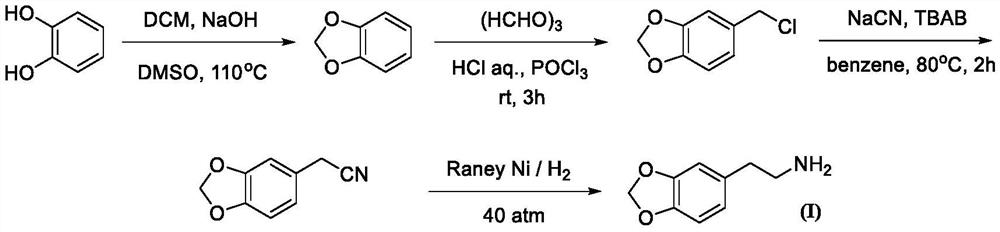
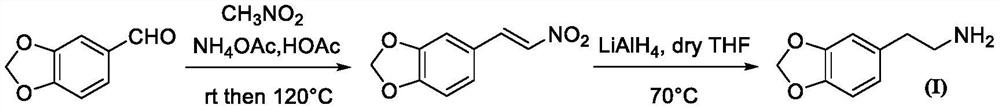
Synthesis method of homopiperony lamine
A technology of piperonyl ethylamine and a synthesis method, applied in the field of organic chemical synthesis, can solve the problems of complicated operation, many synthesis steps, high price and the like, and achieves the effects of mild reaction conditions, high chemical yield and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Embodiment 1 The synthetic method of the first piperethylamine provided by the present invention
[0113] 1.1 Preparation of 3,4-dihydroxymandelic acid (III)
[0114] Under ice-cooling, gradually add catechol (30 g, 272 mmol) into aqueous sodium hydroxide (17.6 g, 441 mmol) (240 mL), and stir until completely dissolved. Then add basic aluminum oxide (11 g) thereto, stir evenly, and then transfer to a 60° C. oil bath for preheating. Under heating at 60°C, an aqueous solution of glyoxylic acid (50wt%, 36.7g) was gradually added dropwise thereto, and the reaction was continued at 60°C for 12 hours after the addition was completed, and the reaction was monitored by TLC. The pH of the reaction solution was adjusted to 3-4 with concentrated hydrochloric acid, and the aqueous layer was extracted with methyl tert-butyl ether, dried over anhydrous sodium sulfate and spin-dried to recover the raw material (7.8 g, conversion rate 74%). The pH of the aqueous phase was adjusted to...
Embodiment 2
[0128] Embodiment 2 The synthetic method of the first piperethylamine provided by the present invention
[0129] 2.1 Preparation of 3,4-dihydroxymandelic acid (III)
[0130] Under ice-cooling, catechol (41 g, 371 mmol) was gradually added to sodium hydroxide (29.4 g, 735 mmol) aqueous solution (615 mL), and stirred until completely dissolved. Then add basic aluminum oxide (28g) thereto, stir evenly, and then transfer to 80°C oil bath for preheating. Under heating at 80°C, an aqueous solution of glyoxylic acid (50 wt%, 36.7g, 247mmol) was gradually added dropwise thereto, and the reaction was continued at 80°C for 18 hours after the addition was completed, and the reaction was monitored by TLC. The pH of the reaction solution was adjusted to 3-4 with concentrated hydrochloric acid, and the aqueous layer was extracted with methyl tert-butyl ether, dried over anhydrous sodium sulfate and then spin-dried to recover the raw material (11.0 g, conversion rate 70%). The pH of the aq...
Embodiment 3
[0144] Embodiment three The synthetic method of the second piperethylamine provided by the present invention
[0145] 3.1 Preparation of pepper ring (III)
[0146] Under ice-cooling, gradually add catechol (22 g, 200 mmol) into aqueous sodium hydroxide (24 g, 600 mmol) (100 mL), and stir until completely dissolved. Then TBAB (6.4g, 20mmol) was added thereto, and dichloromethane (50mL) was added after stirring well. The reaction was heated at 80°C for 24 hours, and the reaction was monitored by TLC. The reaction solution was distilled at atmospheric pressure (100°C) to obtain a water-containing azeotrope, and the piperonine (19.9 g, 82%) was obtained after liquid separation. The product was preserved by molecular sieves with a content of 99% (HPLC).
[0147] 1 H NMR (400MHz, Chloroform-d) δ9.80(s, 1H), 7.40(dd, J=8.0 1.6Hz, 1H), 7.32(d, J=1.6Hz, 1H), 6.92(d, J=8.0 Hz,1H),6.07(s,2H).
[0148] 3.2 Preparation of piperonal (IV)
[0149] At room temperature, piperonylcycline ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com