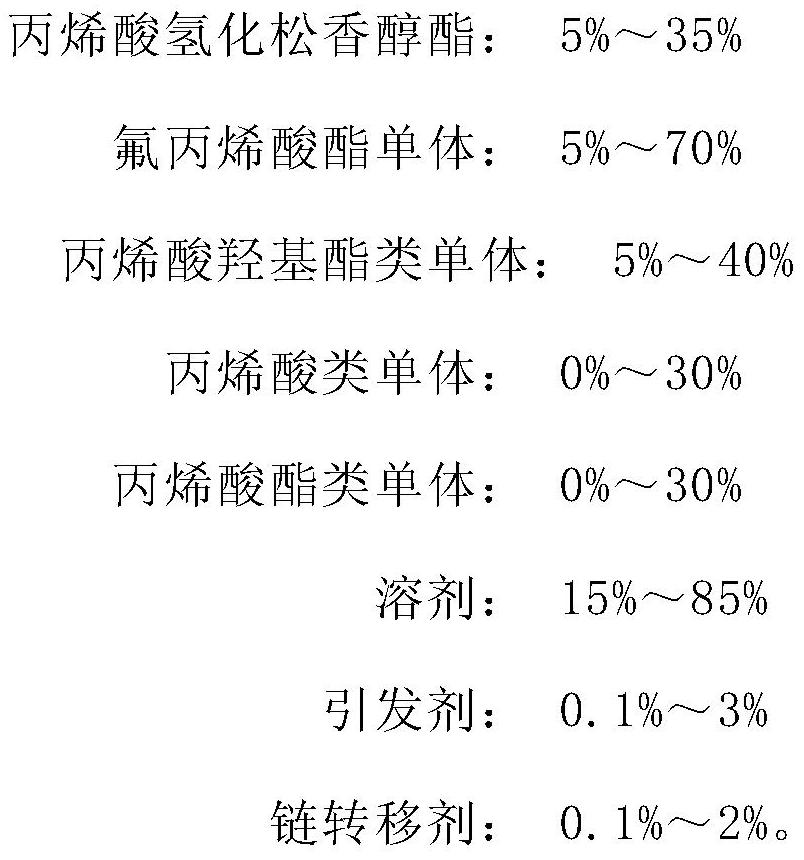
Acrylic acid hydrogenated abietinol ester modified fluorocarbon resin and preparation method thereof
A technology of hydrogenated rosin alcohol acrylate and hydrogenated rosin alcohol is applied in the direction of polyurea/polyurethane coatings, coatings, anti-corrosion coatings, etc. The effect of improving hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Step 1: dissolving hydrogenated abietyl alcohol in dichloromethane to prepare a solution with a mass concentration of 65%;
[0070] Step 2: Add triethylamine accounting for 32% of the mass of hydrogenated abietyl alcohol and 0.2% p-hydroxyanisole to the above hydrogenated abietyl alcohol solution, and after mixing evenly, slowly add 28% of the mass of hydrogenated abietyl alcohol at 45°C % of acryloyl chloride, after the dropwise addition, reflux for 6 hours to complete the reaction, separate and remove the precipitate, wash with deionized water to neutrality, remove the solvent by vacuum extraction, and obtain hydrogenated abietyl alcohol acrylate;
[0071] Step 3: to 20% by mass percentage of hydrogenated abietyl alcohol acrylate, add 30% by mass of ethyl acetate / toluene mixed solvent with a volume ratio of 2:1, 30% by mass of formaldehyde dodecafluoroheptyl acrylate, 10% by mass of hydroxybutyl acrylate, 8.5% by mass of methacrylic acid, 1% by mass of dibenzoyl perox...
Embodiment 2
[0075] Step 1: Dissolving hydrogenated abietyl alcohol in a tetrahydrofuran / trichloroethylene mixed solvent with a volume ratio of 4:1 to prepare a solution with a mass concentration of 50%;
[0076] Step 2: Add N,N-dimethylformamide and 1% 2,5-di-tert-butylhydroquinone accounting for 23% of the mass of hydrogenated abietyl alcohol to the above-mentioned hydrogenated abietyl alcohol solution, mix well, and At 60°C, slowly add acryloyl chloride accounting for 28% of the mass of hydrogenated abietyl alcohol. After the dropwise addition, reflux for 3 hours to complete the reaction, separate and remove the precipitate, wash with deionized water until neutral, and vacuum extract to remove the solvent. Obtain hydrogenated abietyl alcohol ester of acrylate;
[0077] Step 3: To 15% by mass percentage of hydrogenated abietyl alcohol acrylate, add 20% by mass of tetrahydrofuran / butyl acetate mixed solvent with a volume ratio of 1:2, 30% by mass of formazan Hexafluorobutyl acrylate, 15%...
Embodiment 3
[0082] Step 1: dissolving hydrogenated abietyl alcohol in a butyl acetate / petroleum ether mixed solvent with a volume ratio of 2:1 to prepare a solution with a mass concentration of 80%;
[0083] Step 2: Add 13% N,N-dimethylacetamide, 16% triethylamine, 0.5% p-hydroxyanisole, 0.5% 2- After mixing tert-butylhydroquinone evenly, slowly add methacryloyl chloride accounting for 33% of the mass of hydrogenated abietyl alcohol at 35°C, after the dropwise addition, reflux for 5 hours to complete the reaction, separate and remove the precipitate, And after washing with deionized water to neutrality, the solvent is removed by vacuum extraction to obtain hydrogenated abietyl alcohol acrylate;
[0084] Step 3: Add butyl acetate / toluene / tetrahydrofuran mixed solvent with a mass percentage of 15% and a volume ratio of 1:1:1 to the hydrogenated abietyl alcohol acrylate with a mass percentage of 20%, and the mass percentage 10% hexafluorobutyl acrylate, 10% by mass of hexafluorobutyl methac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com