Solid-state electrolyte and solid-state battery comprising same
A solid electrolyte, ionic conductivity technology, applied in the direction of electrolyte immobilization/gelation, secondary batteries, circuits, etc., can solve the problems of volume expansion of positive and negative materials, poor interface compatibility, easy interface separation, etc. The effect of increasing crosslinking density, strong mechanical strength and avoiding material damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068]
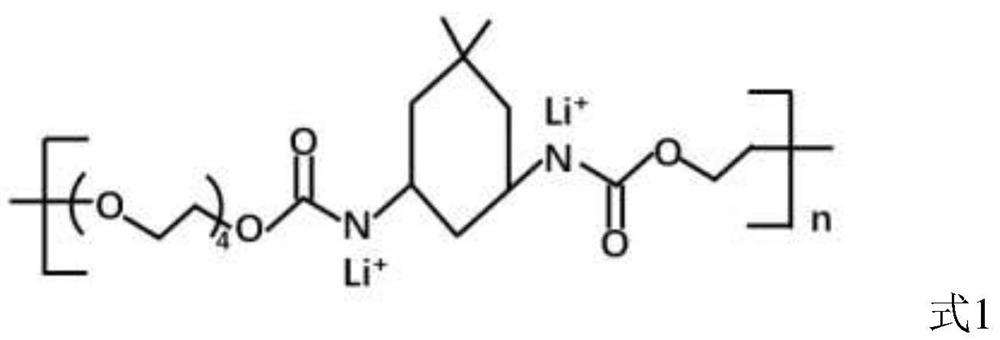
[0069] The present invention also provides a method for preparing a solid electrolyte, comprising the following steps:
[0070] S1: Diisocyanate and alcohol compound are polymerized to obtain a polymer;
[0071] S2: The polymer is subjected to lithiation treatment to obtain a lithiation-treated polymer.
[0072] According to the present invention, the method further comprises the steps of:
[0073] S3: adding an ion conductor to obtain the solid electrolyte.
[0074] According to the present invention, the step S1 specifically includes the following steps:
[0075] S1-1: Dissolve the diisocyanate in an organic solvent and stir for a certain period of time under an argon atmosphere;
[0076] S1-2: Add the alcohol compound and the catalyst to the above solution, and stir at a certain temperature (such as 70-90°C) for a certain period of time (such as 12-48h);
[0077] S1-3: Add alcohol solvent to the above solution, stir for a certain period of time (such as 1-5 h...
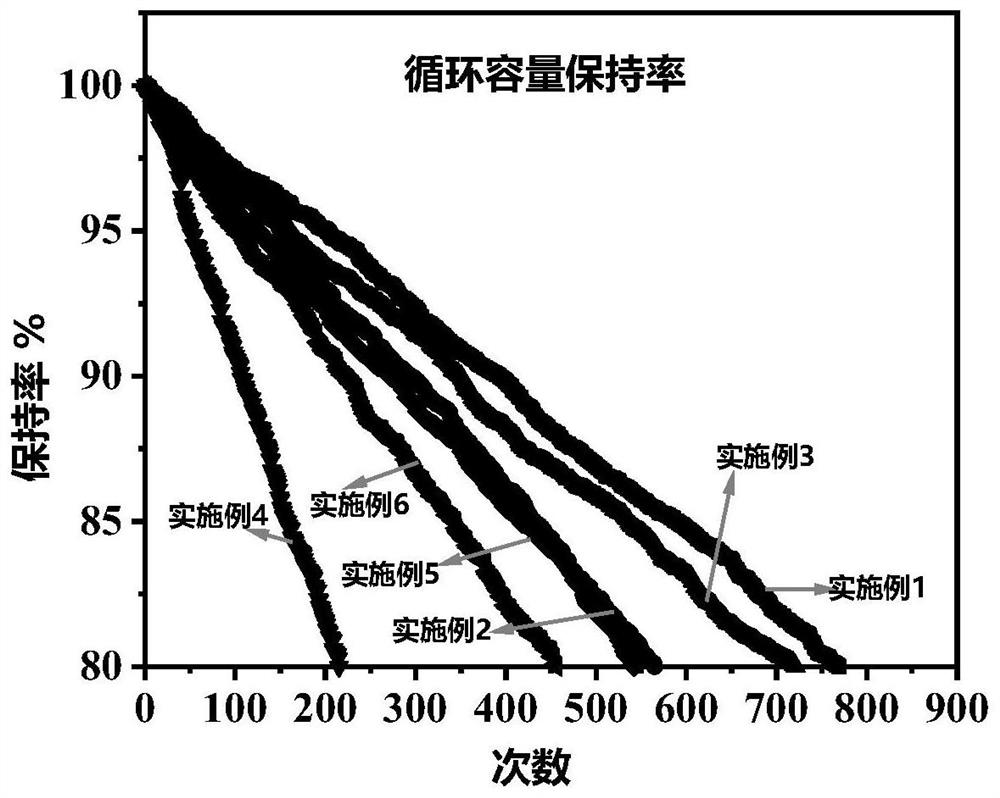
Embodiment 1
[0105] (a) Preparation of solid electrolyte
[0106] (1) 1.5g of isophorone diisocyanate (IPDI) was dissolved in 100ml of anhydrous DMF, and stirred for a certain period of time under an argon atmosphere;
[0107] (2) Add 1.6g of pentylene glycol and a drop of dibutyltin dilaurate (DBTDL) to the above solution, and stir at 80°C for 24h.
[0108] (3) Add 3ml of methanol to the above solution, stir for 1h, and remove excess isocyanate groups.
[0109] (4) Add 3 g of lithium hydride to the above solution, and stir at 80° C. for more than 24 hours to carry out lithiation reaction.
[0110] (5) Add 0.3g lithium salt and 0.2g LLZTO powder to the above solution to improve the ionic conductivity of the solid electrolyte.
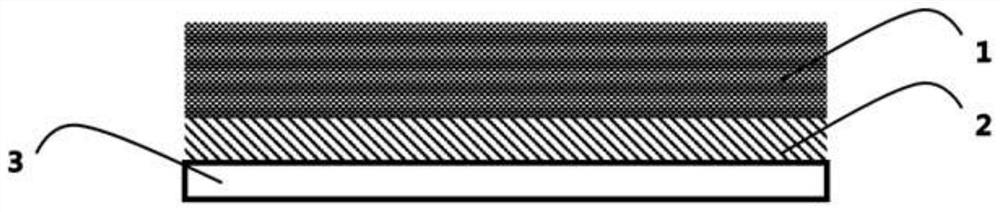
[0111] (6) Introduce the product into a polytetrafluoroethylene mold, place it in a blast oven and dry it at 90°C for 48 hours, then put it in a vacuum oven and dry it at 70°C for 24 hours to remove the residual solvent to obtain a solid electrolyte. The thickness...
Embodiment 2
[0120] Other operations are the same as in Example 1, the difference is that the preparation process of the solid electrolyte is different:
[0121] (1) Dissolve 1.5g of xylene diisocyanate (MPI) in 100ml of anhydrous DMF, and stir for a certain period of time under an argon atmosphere;
[0122] (2) Add 1.6g of pentylene glycol and a drop of dibutyltin dilaurate (DBTDL) to the above solution, and stir at 80°C for 24h.
[0123] (3) Add 3ml of methanol to the above solution, stir for 1h, and remove excess isocyanate groups.
[0124] (4) Add 3 g of lithium hydride to the above solution, and stir at 80° C. for more than 24 hours to carry out lithiation reaction.
[0125] (5) Add 0.3g lithium salt and 0.2g LLZTO powder to the above solution to improve the ionic conductivity of the solid electrolyte.
[0126] (6) The product was introduced into a polytetrafluoroethylene mold, placed in a blast oven to dry at 90°C for 48 hours, and then placed in a vacuum oven to dry at 70°C for 24...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com