Synthesis method and purification method of semaglutide protected amino acid
A technology of semaglutide and a synthesis method, which is applied in the field of biomedicine, can solve the problems of poor protection effect of tert-butanol, difficult separation and purification of products, and difficulty in complete reaction, and achieves low cost, short operation time, and reaction speed. quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
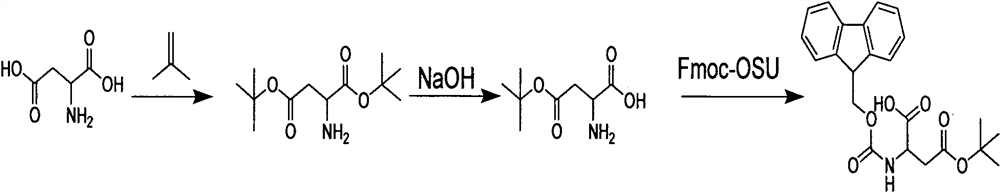
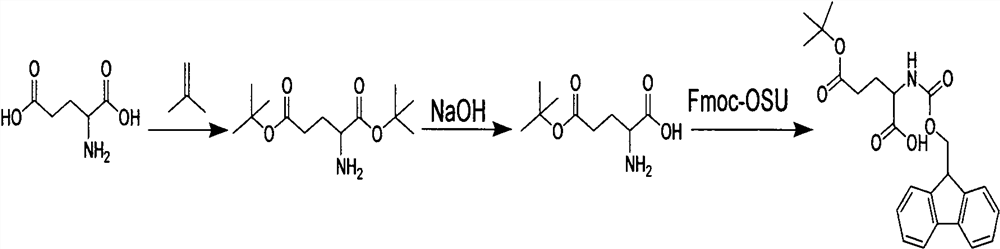
[0021] A synthetic method for semaglutide protected amino acids, comprising the steps of:
[0022] Step 1, the amino acid is under the condition of dioxane solvent, stirring and adding sulfuric acid solution and isobutylene, and completely reacting to obtain intermediate I;
[0023] Step 2. Cool the reaction solution in the previous step to below 0°C in an ice bath, add sodium hydroxide solution, adjust the pH to 9-10, and react completely after stirring to obtain intermediate II.
[0024] Step 3. Keep the reaction solution in the previous step under ice bath conditions, add the dioxane solution of Fmoc-osu, the reaction is complete, acidify the pH to 3-4 with dilute hydrochloric acid, extract the aqueous phase with an organic solvent, and dry the extracted organic phase. Spin dry to get the product.
[0025] In step one, the amino acid is aspartic acid or glutamic acid. Sulfuric acid is one of strong acids, in the present invention, adopt sulfuric acid as strong acid, mainl...
Embodiment 2
[0030] The purification method of the semaglutide protected amino acid of embodiment 1, comprises the steps:
[0031] Step 1, adding the semaglutide-protected amino acid obtained by the above preparation method into concentrated hydrochloric acid, stirring and dissolving at 70-100 degrees Celsius to obtain a hydrochloride solution; cooling and crystallizing at 15-25 degrees Celsius;
[0032] Step 2, add methanol or acetonitrile solvent, stir at 70-100 degrees Celsius, wash, and filter to obtain a high-purity hydrochloride solution,
[0033] Step 3, add methanol or acetonitrile solvent to dissolve, then add propylene oxide to remove hydrochloric acid, filter, and wash with ethyl acetate, petroleum ether or dichloromethane to obtain high-purity semaglutide-protected amino acids.
Embodiment 3
[0035] Determine its optimum synthesis condition by contrast experiment: in the synthesis of Fmoc-Asp(Otbu)-OH, investigate the influences such as different reaction temperature, molar ratio, reaction time etc. to reaction, improve product yield and purity, make process The conditions have reached the advanced level at home and abroad.
[0036] 1.1 Synthetic route
[0037]
[0038] 1.2 Specifications of main raw materials
[0039] raw material name content(%) Specification 1 Aspartic acid 98 industrial grade 2 Isobutylene 98 industrial grade 3 sodium hydroxide 99 industrial grade 4 sulfuric acid 99 industrial grade 5 Dioxane 98 industrial grade 6 Anhydrous Sodium Sulfate 96 industrial grade 7 ethyl acetate 98 industrial grade 8 Fmoc-osu 98 industrial grade
[0040] 1.3 Operation steps
[0041] (1)H 2 Synthesis of N-Asp(otbu)-Otbu
[0042] In a 500ML three-neck flask, add 26.6g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com