Production method of linear alkylbenzene
A technology of linear alkylbenzene and production method, applied in chemical instruments and methods, addition of unsaturated hydrocarbons and saturated hydrocarbons to hydrocarbon production, including molecular sieve catalysts, etc. and product selectivity, etc., to achieve the effect of improving reaction activity and product selectivity, long catalyst life, reducing reaction temperature and reaction pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add 1.25g of sodium hydroxide into 150g of deionized water, stir to dissolve. Add 5.5g of sodium metaaluminate and stir to dissolve. Stirring was continued vigorously for 1 h. Slowly add 50g of tetraethylammonium hydroxide solution and continue to stir vigorously for 1h. Slowly add 100g of silica sol, and continue to stir vigorously for 2h to obtain a crystallized gel. The crystallized gel was crystallized at 160° C. for 24 hours, and cooled to 140° C. to continue crystallization for 12 hours. The crystallized product is washed, filtered and dried to obtain the beta zeolite molecular sieve. After calcination at 550°C for 6 hours to remove the template agent, ammonium exchange with 1mol / L ammonium nitrate solution at 80°C for 2 hours, and calcination at 550°C for 4 hours to obtain H-type β zeolite molecular sieve.
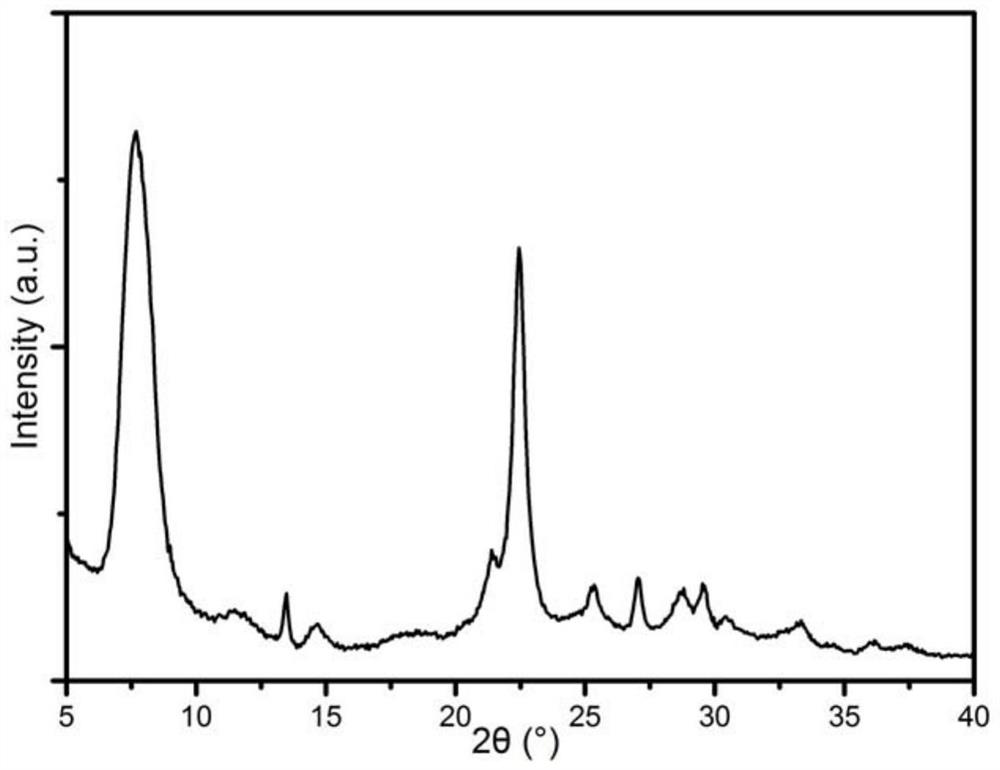
[0049] Among them, the XRD spectrum of the H-type beta zeolite molecular sieve is shown in figure 1 , SEM image see figure 2 . Depend on figure 1 an...
Embodiment 2
[0054] Add 1.57g of potassium hydroxide into 200g of deionized water, stir to dissolve. Add 16.5g of aluminum sulfate and stir to dissolve. Stirring was continued vigorously for 1 h. Slowly add 21.5g of tetraethylammonium bromide and continue to stir vigorously for 1h. Slowly add 45g of white carbon black and continue to stir vigorously for 3h to obtain a crystallized gel. The crystallized gel was crystallized at 170°C for 12h, and cooled to 125°C for 24h. The crystallized product is washed, filtered and dried to obtain the beta zeolite molecular sieve. After calcination at 550°C for 6 hours to remove the template agent, ammonium exchange with 1mol / L ammonium nitrate solution at 80°C for 2 hours, and calcination at 550°C for 4 hours to obtain H-type β zeolite molecular sieve.
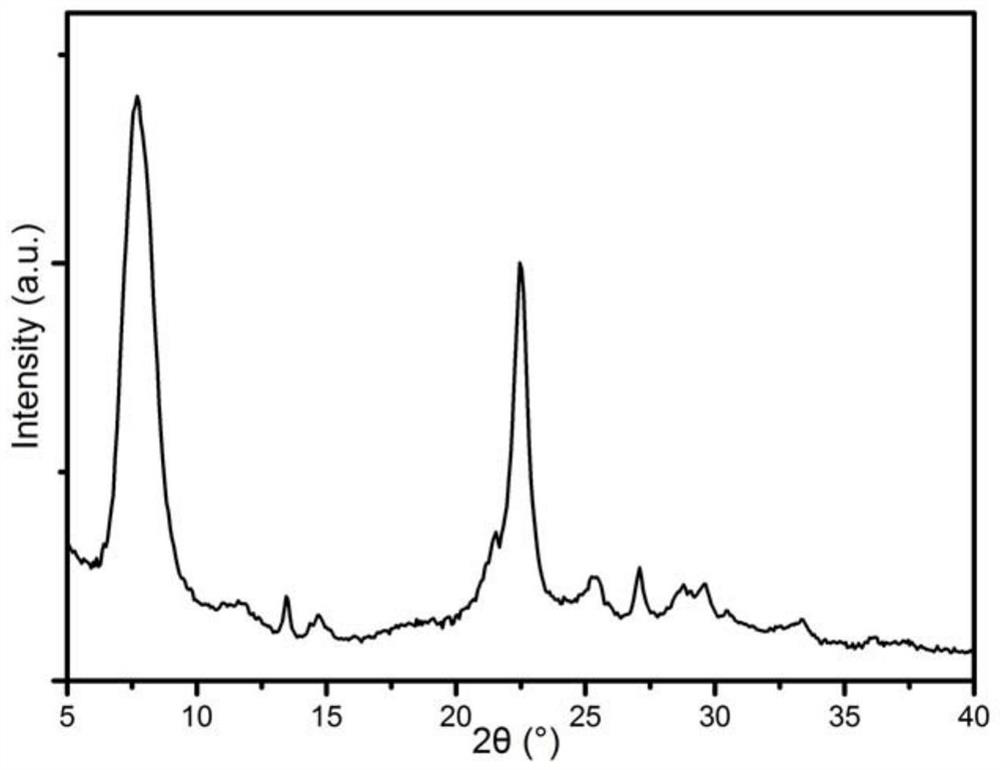
[0055] Among them, the XRD spectrum of the H-type beta zeolite molecular sieve is shown in image 3 , SEM image see Figure 4 . Depend on image 3 and Figure 4 It can be seen that the H-type b...
Embodiment 3
[0060] Take 2 g of the finished catalyst product prepared in Example 1, put it into a fixed-bed reactor, and feed the mixture of benzene and 1-decene. The reaction conditions are: benzene-ene ratio 12, reaction temperature 137°C, reaction pressure 5.0MPa, mass space velocity 4.0.
[0061] The reaction result is: after 200 hours of reaction, the conversion rate of 1-decene is 99.23%, the selectivity of 2-alkylbenzene is 49%, and the selectivity of 2-alkylbenzene and 3-alkylbenzene is 63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com