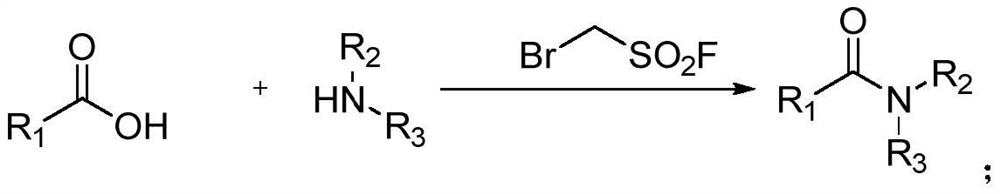
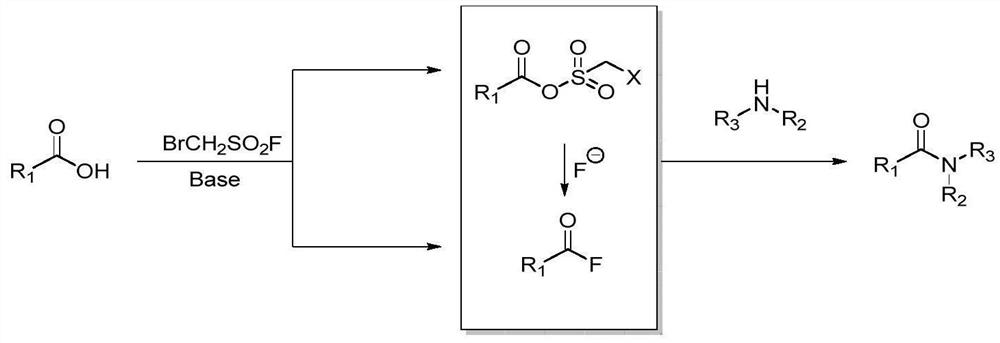
Preparation method of amide compound
A technology of dehydration and condensation of amide compounds, applied in the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve the problems of high cost of coupling reagents, many chemical wastes, cumbersome procedures, etc., and achieve easy separation Purification, broad substrate range, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of preparation method of amides compound, reaction formula is as follows:
[0025]
[0026] Specific steps are as follows:
[0027] Add diethylamine (10mmol), benzoic acid (30mmol), sodium hydroxide (30mmol), bromomethylsulfonyl fluoride (15mmol), and acetonitrile (25mL) into a 100mL pressure bottle, and reflux the reaction in an oil bath at 50°C 18h, after the reaction, add 30mL of 1M sodium hydroxide solution to the reaction solution, then extract 3 times with 20mL of ethyl acetate, wash the extract with 20mL of 1M hydrochloric acid solution, then add anhydrous sodium sulfate to dry and distill under reduced pressure, The white solid N,N-diethylbenzamide fluoride (1.41 g, 80% yield) was obtained.
[0028] The hydrogen spectrum data of the amide compounds obtained in the present embodiment are as follows: 1 H-NMH (500MHz, CDCl 3 )δ=7.40-7.36(m,5H),3.56(s,2H),3.26(s,2H),1.26(s,3H),1.11(s,3H).
Embodiment 2
[0030] A kind of preparation method of amides compound, reaction formula is as follows:
[0031]
[0032] Specific steps are as follows:
[0033] Add aniline (10mmol), o-trifluoromethylbenzoic acid (30mmol), sodium hydroxide (30mmol), bromomethylsulfonyl fluoride (15mmol), acetonitrile (25mL) into a 100mL pressure bottle, and place in an oil bath at 50°C Under reflux for 18 hours, after the reaction, add 30mL of 1M sodium hydroxide solution to the reaction solution, then extract 3 times with 20mL of ethyl acetate, wash the extract with 20mL of 1M hydrochloric acid solution, then add anhydrous sodium sulfate to dry and reduce Pressure distillation gave white solid N-phenyl-2-(trifluoromethyl)benzamide (2.20 g, 83% yield).
[0034] The hydrogen spectrum data of the amide compounds obtained in the present embodiment are as follows: 1 H-NMH (500MHz, DMSO-d 6 )δ=10.55(s,1H),7.85(d,J=7.6Hz,1H),7.80(t,J=7.3Hz,1H),7.73-7.70(m,4H),7.36(t,J=7.6 Hz,2H),7.12(t,J=7.3Hz,1H). 19 F-NM...
Embodiment 3
[0036] A kind of preparation method of amides compound, reaction formula is as follows:
[0037]
[0038] Specific steps are as follows:
[0039] Add L-valine ethyl ester (10mmol), benzoic acid (30mmol), sodium hydroxide (30mmol), bromomethylsulfonyl fluoride (15mmol), acetonitrile (25mL) to a 100mL pressure bottle, The reaction was refluxed in the bath for 18 hours. After the reaction, 30 mL of 1M sodium hydroxide solution was added to the reaction solution, and then extracted three times with 20 mL of ethyl acetate. The extract was washed with 20 mL of 1M hydrochloric acid solution, and then dried by adding anhydrous sodium sulfate. Distilled under reduced pressure to obtain white solid benzoyl-L-valine ethyl ester (2.17g, 87% yield, 99% ee).
[0040] The hydrogen spectrum data of the amide compounds obtained in the present embodiment are as follows: 1 H-NMR (500MHz, CDCl 3 )δ=7.81(d, J=7.3Hz, 2H), 7.50(t, J=7.3Hz, 1H), 7.44(t, J=7.5Hz, 2H), 6.68(d, J=7.7Hz, 1H) ,4.76...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com