Method for determining related substances in fosfomycin sodium by using nuclear magnetic resonance hydrogen spectrum
A technology of proton nuclear magnetic resonance spectroscopy and related substances, which is applied to the analysis of nuclear magnetic resonance, can solve the problems of long analysis time, loss of water-soluble components, loss of chromatographic separation, etc., and achieves simple operation, rapid measurement and good reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] (1Z)-prop-1-en-1-yl phosphonate sodium, propyl phosphonate sodium, 2-propenyl phosphonate sodium and (1,2-dihydroxypropyl ) The mensuration of sodium phosphonate comprises the following steps:
[0064] The first step: the preparation of the first mixed solution: Accurately weigh 470.5 mg of fosfomycin sodium sample II with a mass fraction of 99.3% and 190.1 mg of 4-bromopyridine hydrochloride with a mass fraction of 90%, dissolve in 10 ml D 2 O, the first mixed solution was obtained;
[0065] Step 2: Preparation of test solution: Accurately measure 0.5ml of the first mixed solution, add 10ml of D 2 O is diluted to obtain the test solution (the test solution containing 23.36mg fosfomycin sodium and 8.55mg mass fraction is 90% 4-bromopyridine hydrochloride);
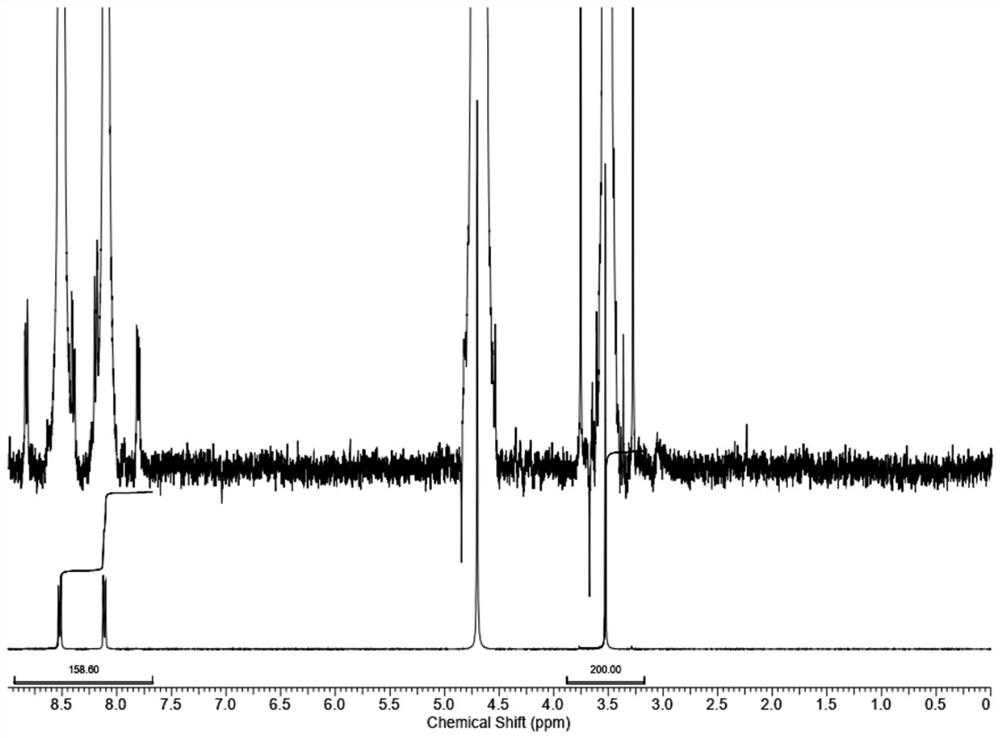
[0066] The third step: proton nuclear magnetic resonance spectrum test: the test procedure is the same as that of the blank control example. Record the spectrum. The internal standard and the characteristic peak...
Embodiment 2
[0077] (1Z)-prop-1-en-1-yl phosphonate sodium, propyl phosphonate sodium, 2-propenyl phosphonate sodium and (1,2-dihydroxypropyl ) The mensuration of sodium phosphonate comprises the following steps:
[0078] The first step: the preparation of test solution: accurately measure the first mixed solution 0.5ml in embodiment one, add 10mlD 2 O is diluted to obtain the second mixed solution; adding 0.1ml to the second mixed solution containing 20ml of propylphosphonic acid 10.1mg with a mass fraction of 95% 2 O solution, to get the test solution (the test solution containing 23.36mg fosfomycin sodium and 8.55mg mass fraction is 90% 4-bromopyridine hydrochloride);
[0079] The remaining steps are the same as in Embodiment 1.
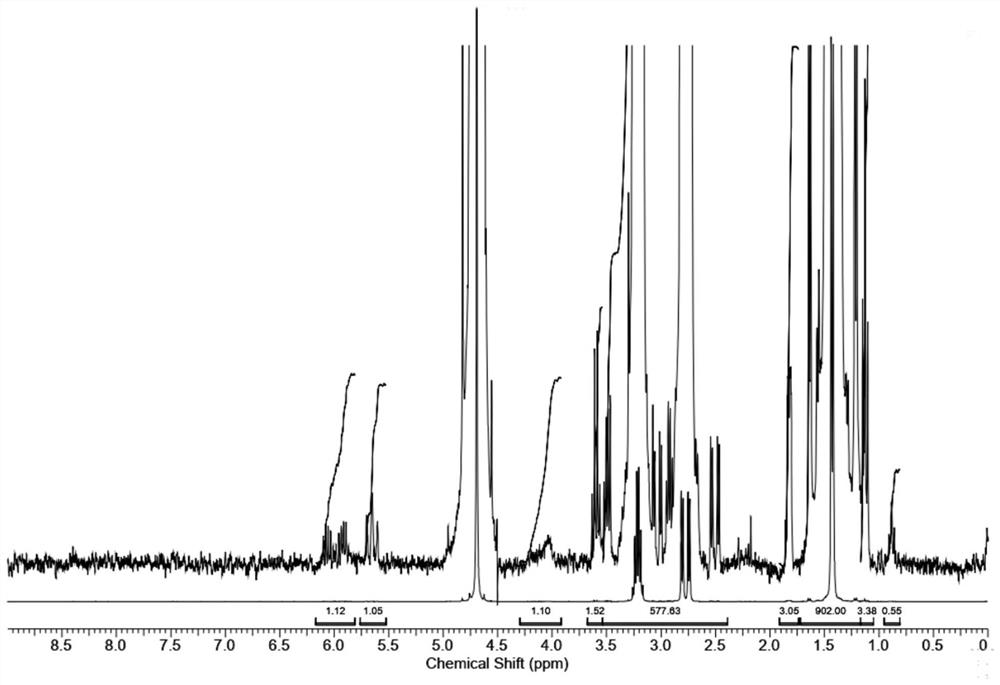
[0080] The amount of propylphosphonic acid with a mass fraction of 95% in the test solution of Example 2 is 0.05 mg.
[0081] In the second embodiment 1 H NMR spectrum as Figure 4 shown.
Embodiment 3
[0083] (1Z)-prop-1-en-1-yl phosphonate sodium, propyl phosphonate sodium, 2-propenyl phosphonate sodium and (1,2-dihydroxypropyl ) The mensuration of sodium phosphonate comprises the following steps:
[0084] The first step: the preparation of test solution: accurately measure the first mixed solution 0.5ml in embodiment one, add 10mlD 2 O is diluted to obtain the second mixed solution; in the second mixed solution, adding 0.2ml containing 20ml of propylphosphonic acid 10.1mg with a mass fraction of 95% 2 O solution, to get the test solution (the test solution containing 23.36mg fosfomycin sodium and 8.55mg mass fraction is 90% 4-bromopyridine hydrochloride);
[0085] The remaining steps are the same as in Embodiment 1.
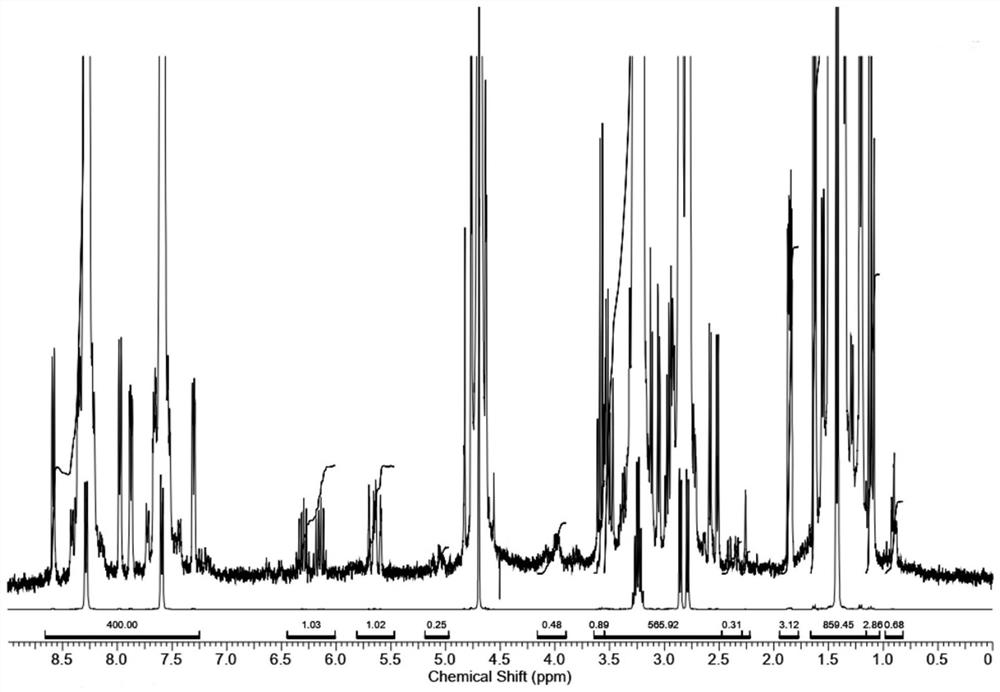
[0086] The amount of propylphosphonic acid with a mass fraction of 95% in the test solution of Example 3 was 0.10 mg.
[0087] In the third embodiment 1 H NMR spectrum as Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com