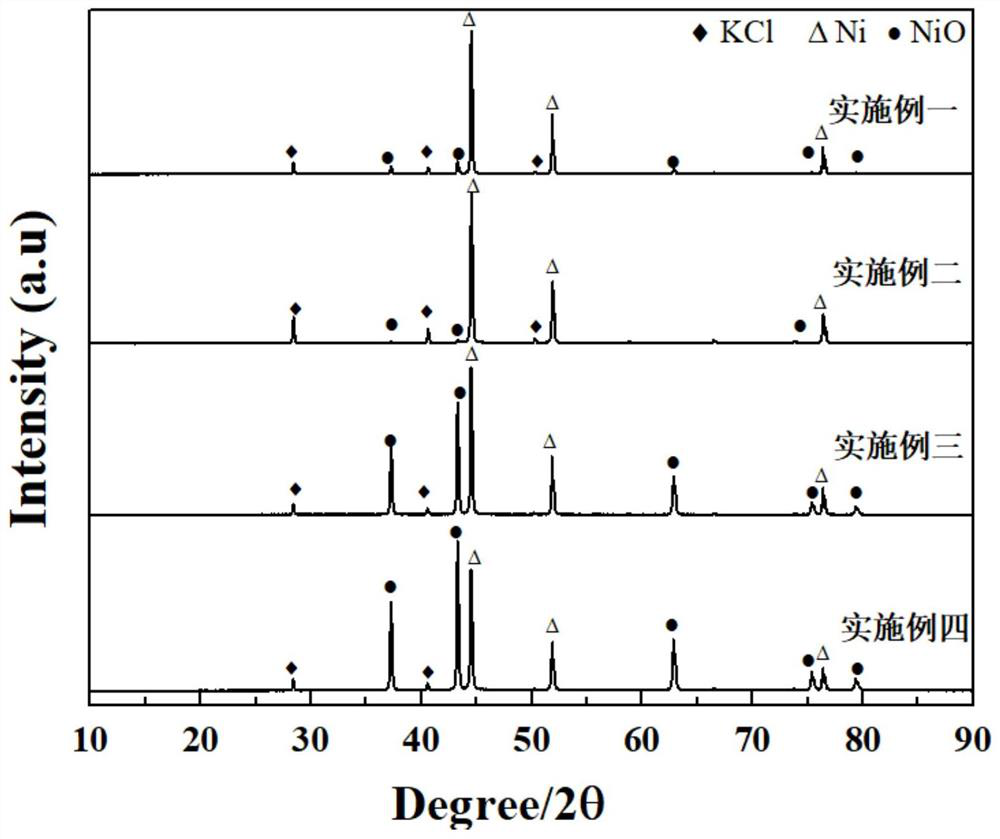
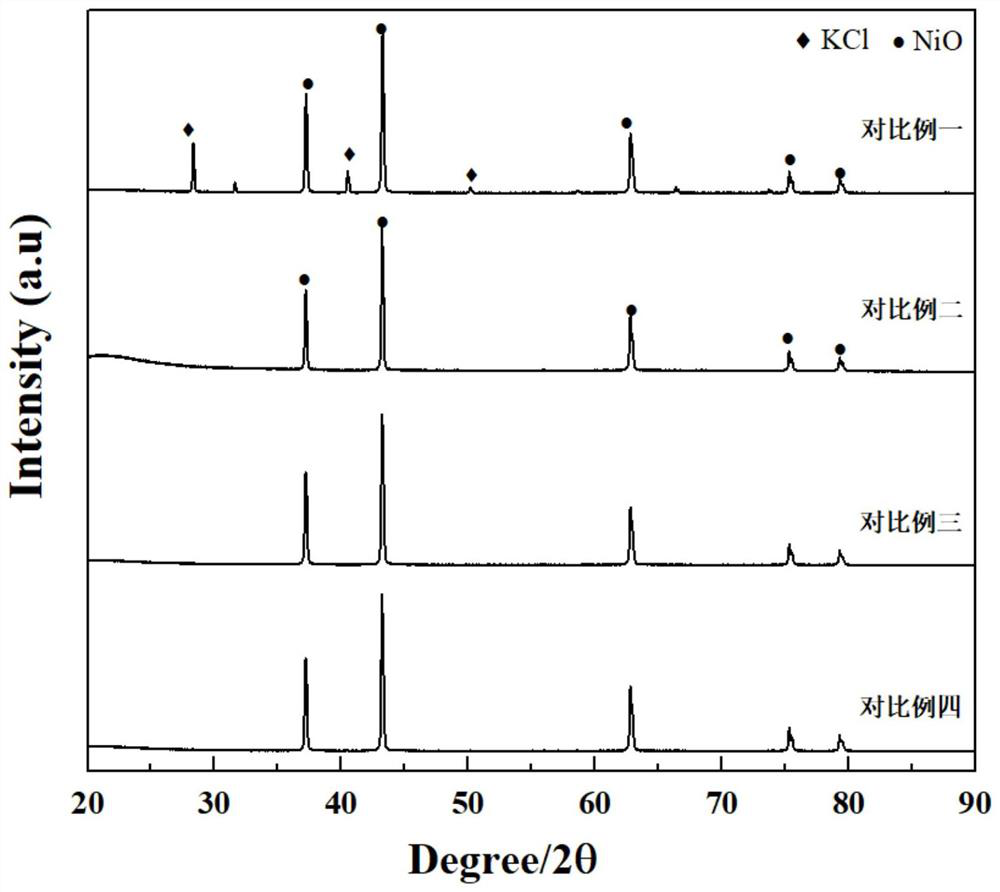
NiO/Ni bifunctional electrolyzed water catalyst and preparation method thereof
A technology for electrolyzing water and catalysts, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., which can solve the problems of high cost, increased complexity of electrolyzed water equipment, and increased work To achieve the effect of increasing efficiency, excellent total water splitting activity, and improving energy conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh a certain amount of NiO powder obtained from Comparative Example 1, and transfer it to a crucible, place the crucible in a tube furnace, and use H 2 Partially restore it. Before raising the temperature, a mixture of hydrogen and argon (Ar: 95%, H 2 :5%) 30min, gas flow rate 100mL min -1 , to remove the air in the tube; then adjust the gas flow rate to 10mL min -1 , raise the temperature of the tube furnace to 450°C and keep it at this temperature for 30min with a heating rate of 10°C / min. After the tube furnace is cooled to room temperature, turn off the gas and take out the sample to obtain NiO / Ni. After mixing the NiO / Ni powder with DMF and PVDF, it is drip-coated on 2*1cm carbon fiber paper (CFP), and it can be used as an electrolytic water electrode material after it is naturally dried, and it is used in alkaline conditions ( Electrochemical HER and OER tests were performed with an electrochemical workstation under 1.0M KOH). Graphite electrode rod and pla...
Embodiment 2
[0038] Weigh a certain amount of NiO powder obtained from Comparative Example 2, and transfer it to a crucible, place the crucible in a tube furnace, and use H 2 Partially restore it. Before raising the temperature, a mixture of hydrogen and argon (Ar: 95%, H 2 :5%) 30min, gas flow rate 100mL min -1 , to remove the air in the tube; then adjust the gas flow rate to 10mL min -1 , raise the temperature of the tube furnace to 450°C and keep it at this temperature for 10 minutes, the heating rate is 10°C / min, after the tube furnace is cooled to room temperature, turn off the gas, and take out the sample to obtain NiO / Ni. After mixing the NiO / Ni powder with DMF and PVDF, it is drip-coated on 2*1cm carbon fiber paper (CFP), and it can be used as an electrolytic water electrode material after it is naturally dried, and it is used in alkaline conditions ( Electrochemical HER and OER tests were performed with an electrochemical workstation under 1.0M KOH). Graphite electrode rod and...
Embodiment 3
[0040] Weigh a certain amount of NiO powder obtained from Comparative Example 2, and transfer it to a crucible, place the crucible in a tube furnace, and use H 2 Partially restore it. Before raising the temperature, a mixture of hydrogen and argon (Ar: 95%, H 2 :5%) 30min, gas flow rate 100mL min -1 , to remove the air in the tube; then adjust the gas flow rate to 20mL·min -1 , raise the temperature of the tube furnace to 450°C and keep it at this temperature for 1min, the heating rate is 5°C / min, after the tube furnace is cooled to room temperature, turn off the gas, and take out the sample to obtain NiO / Ni. After mixing the NiO / Ni powder with DMF and PVDF, it is drip-coated on 2*1cm carbon fiber paper (CFP), and it can be used as an electrolytic water electrode material after it is naturally dried, and it is used in alkaline conditions ( 1.0M KOH) was used to perform electrochemical HER and OER tests on an electrochemical workstation, respectively using graphite electrode...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com