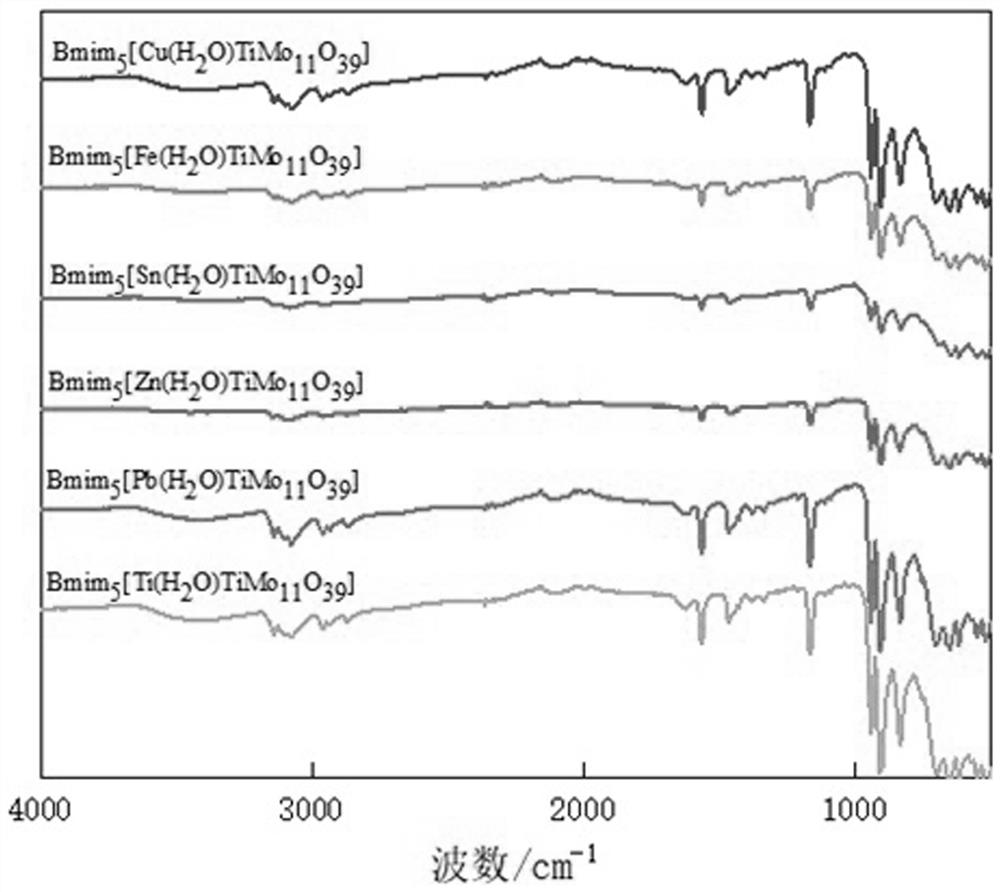
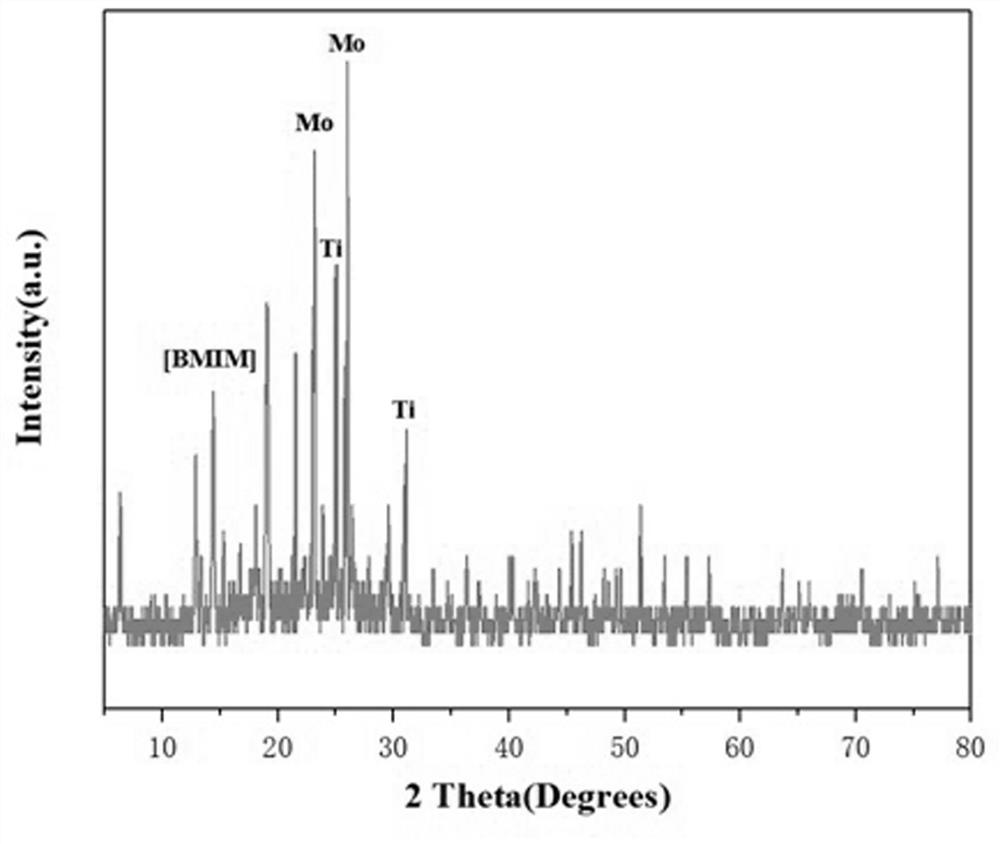
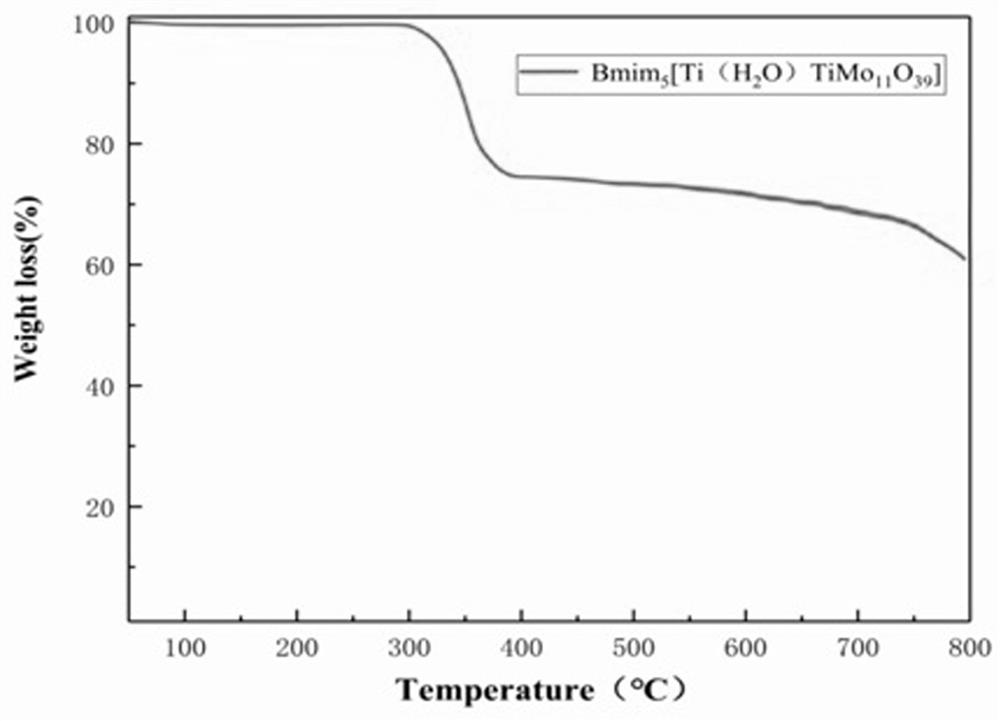
Preparation method and application of titanium heteropolyacid ionic liquid catalyst
A technology of heteropolyacid anions and ionic liquids, which is applied in the direction of catalytic reaction, organic carbonate preparation, chemical instruments and methods, etc., can solve the problems of easy deactivation, easy generation of by-products, poor activity, etc., and achieve non-combustible, good Effects of thermal and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of intermediate A-imidazole ionic liquid
[0035] Weigh a certain amount of N-methylimidazole in the reactor, slowly add a certain amount of chlorobutane dropwise at a rate of 10-100mL / s under the condition of condensing and reflux, and control the reaction temperature at 70°C. After 26 hours of reaction, the The reaction solution was washed and filtered with acetonitrile for 4 times, and dried at 70°C for 8 hours by rotary evaporation to obtain light yellow intermediate A.
[0036] (2) Preparation of intermediate B-metal substituted titanium-based vacancy Keggin-type heteropolyacids
[0037] Weigh a certain amount of sodium molybdate and dissolve it in an appropriate amount of distilled water, heat to a slight boil, adjust the pH to 4, condense and reflux, and slowly add a certain amount of TiCl dropwise at a rate of 10-100ml / s 4Hydrochloric acid aqueous solution, after 2 hours of reaction after the dropwise addition, slowly drop a certain amount of Sn...
Embodiment 2
[0041] (1) Preparation of intermediate A-imidazole ionic liquid
[0042] Weigh a certain amount of N-methylimidazole in the reactor, slowly add a certain amount of chlorobutane dropwise at a rate of 10-100mL / s under reflux conditions, and control the reaction temperature at 90°C. After 22 hours of reaction, the The reaction solution was washed and filtered with acetonitrile for 5 times, and then dried at 90°C for 5 hours by rotary evaporation to obtain light yellow intermediate A.
[0043] (2) Preparation of intermediate B-metal substituted titanium-based vacancy Keggin-type heteropolyacids
[0044] Weigh a certain amount of sodium molybdate and dissolve it in an appropriate amount of distilled water, heat it to a slight boil, adjust the pH to 5, condense and reflux, and slowly add a certain amount of TiCl dropwise at a rate of 10-100ml / s 4 Hydrochloric acid aqueous solution, after the reaction for 2 hours after the dropwise addition, slowly drop a certain amount of zinc acet...
Embodiment 3
[0048] (1) Preparation of intermediate A-imidazole ionic liquid
[0049] Weigh a certain amount of N-methylimidazole in the reactor, slowly add a certain amount of chlorobutane dropwise at a rate of 10-100mL / s under the condition of condensing and reflux, and control the reaction temperature at 80°C. After 24 hours of reaction, the The reaction solution was washed and filtered with acetonitrile for 3 times, and dried at 80°C for 6 hours by rotary evaporation to obtain light yellow intermediate A.
[0050] (2) Preparation of intermediate B-metal substituted titanium-based vacancy Keggin-type heteropolyacids
[0051] Weigh a certain amount of sodium molybdate and dissolve it in an appropriate amount of distilled water, heat it to a slight boil, adjust the pH to 5, condense and reflux, and slowly add a certain amount of TiCl dropwise at a rate of 10-100ml / s 4 Hydrochloric acid aqueous solution, after 2 hours of reaction after the dropwise addition, slowly drop a certain amount o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com