Preparation method of ethyl 2-cyanopropionate
A technology of ethyl cyanopropionate and ethyl cyanoacetate, applied in the field of compound preparation, can solve problems such as unfavorable industrialized production, equipment corrosion, etc., and achieves a technology suitable for large-scale production, reducing equipment corrosion, and simple and easy process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method for ethyl 2-cyanopropionate, comprising the following steps:
[0029] (1) Add 11.3g of ethyl cyanoacetate, 83mL of N,N-dimethylformamide, 16g of 37wt% formaldehyde solution and 0.12g of Pd / C catalyst with a palladium loading of 1% to the autoclave;
[0030] (2) Introduce nitrogen to replace the air, then infuse hydrogen to a pressure of 2.0MPa, heat to a temperature of 60°C and stir to react;
[0031] (3) After the reaction is monitored by gas chromatography, filter and recover the catalyst. The filtrate is washed with 100 mL of water, separated, the organic phase is collected, dried, concentrated, and distilled under reduced pressure at 90~100 °C and 20 mmHg to obtain a colorless liquid 9.54 g, namely ethyl 2-cyanopropionate, the yield is 75%, and the gas phase purity is >99%.
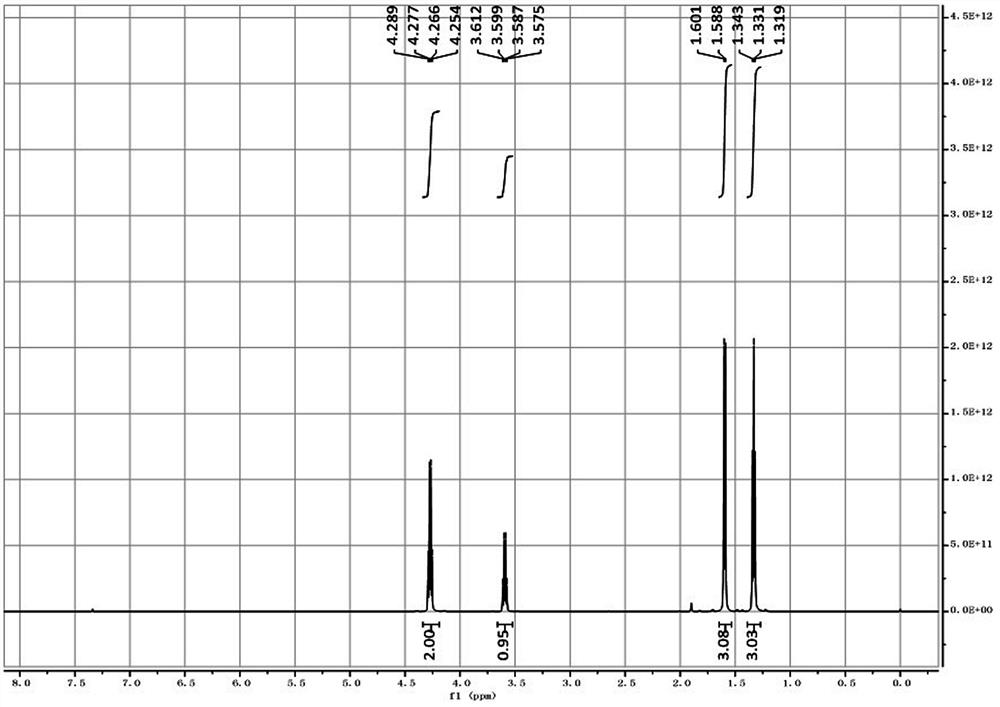
[0032] The proton nuclear magnetic spectrum of the 2-cyanopropionate ethyl ester that present embodiment prepares sees figure 1 , its chemical shift δ 4.27 (q, J =4...
Embodiment 2
[0036] A preparation method for ethyl 2-cyanopropionate, comprising the following steps:
[0037] (1) Add 11.3g of ethyl cyanoacetate, 105mL of N,N-dimethylformamide, 24g of 37wt% formaldehyde solution and 0.06g of Pd / C catalyst with palladium loading of 0.5% to the autoclave;
[0038] (2) Introduce nitrogen to replace the air, then infuse hydrogen to a pressure of 4.0MPa, heat to a temperature of 80°C and stir to react;
[0039] (3) After the reaction is monitored by gas chromatography, filter and recover the catalyst. The filtrate is washed with 100 mL of water, separated, the organic phase is collected, dried, concentrated, and distilled under reduced pressure at 80~90 °C and 14 mmHg to obtain a colorless liquid 10.3g, which is ethyl 2-cyanopropionate, the yield is 81%, and the gas phase purity is >99%.
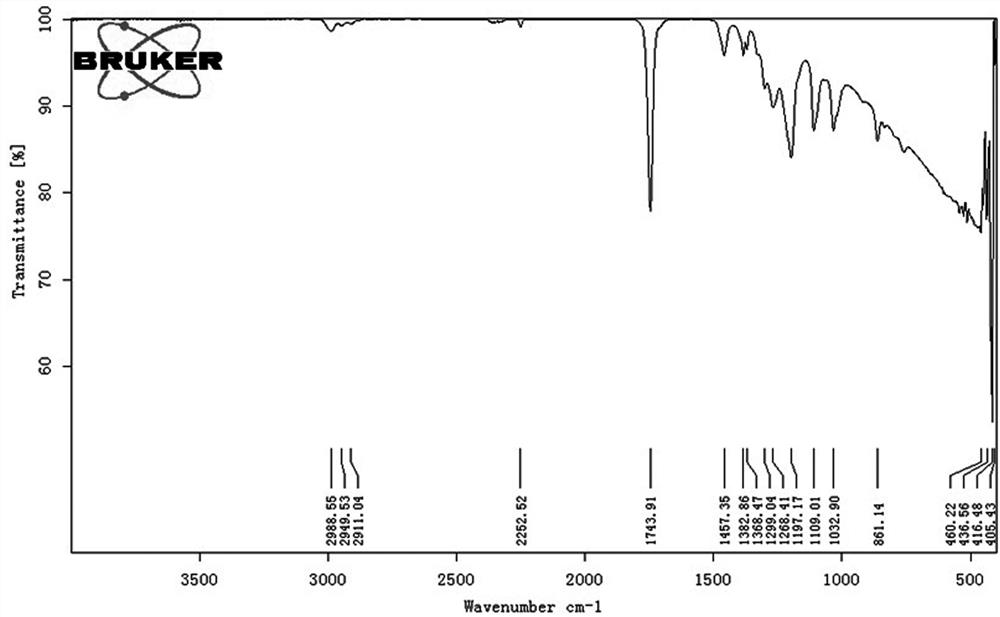
[0040] Product nuclear magnetic hydrogen and infrared spectrograms are similar to those of Example 1.
Embodiment 3
[0042] A preparation method for ethyl 2-cyanopropionate, comprising the following steps:
[0043] (1) Add 11.3g of ethyl cyanoacetate, 105mL of N,N-dimethylformamide, 6.01g of paraformaldehyde and 0.12g of Pd / C catalyst with a palladium loading of 1% to the autoclave;
[0044] (2) Introduce nitrogen to replace the air, then infuse hydrogen to a pressure of 4.0MPa, heat to a temperature of 80°C and stir to react;
[0045] (3) After the reaction is monitored by gas chromatography, filter and recover the catalyst. The filtrate is washed with 100 mL of water, separated, the organic phase is collected, dried, concentrated, and distilled under reduced pressure at 86~102 °C and 17 mmHg to obtain a colorless liquid 11.4g, which is ethyl 2-cyanopropionate, the yield is 90%, and the gas phase purity is >99%.
[0046] Product nuclear magnetic hydrogen and infrared spectrograms are similar to those of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com