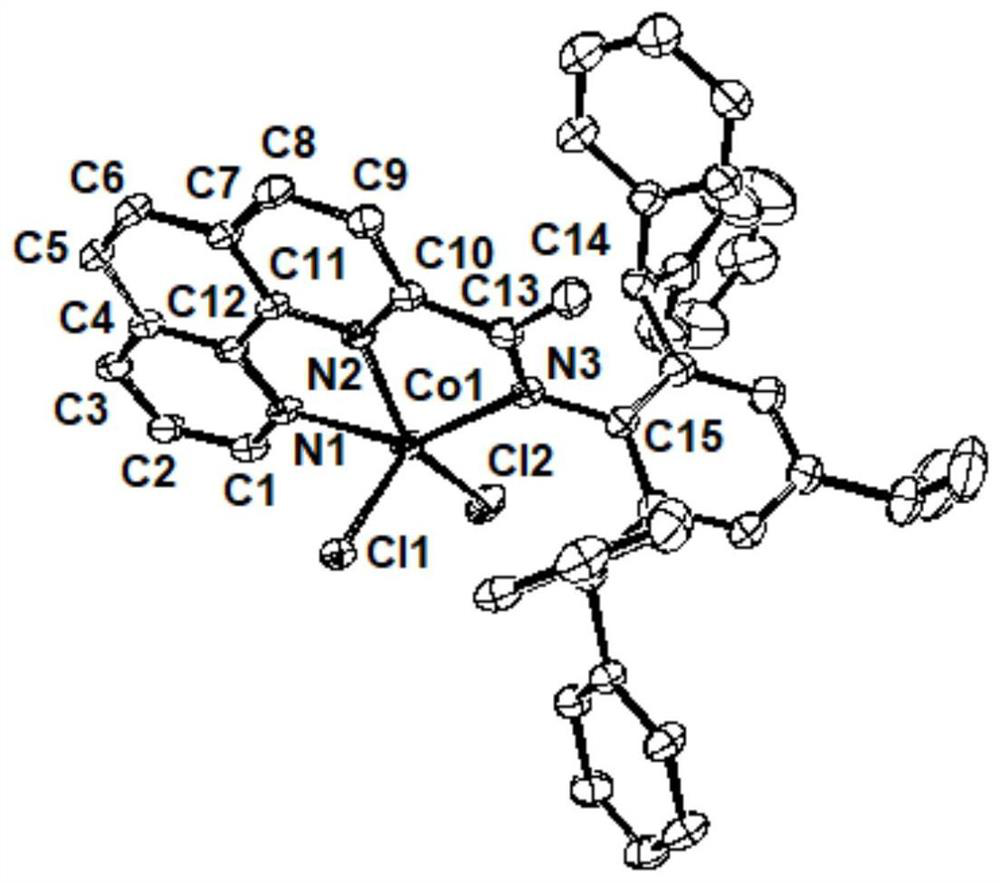
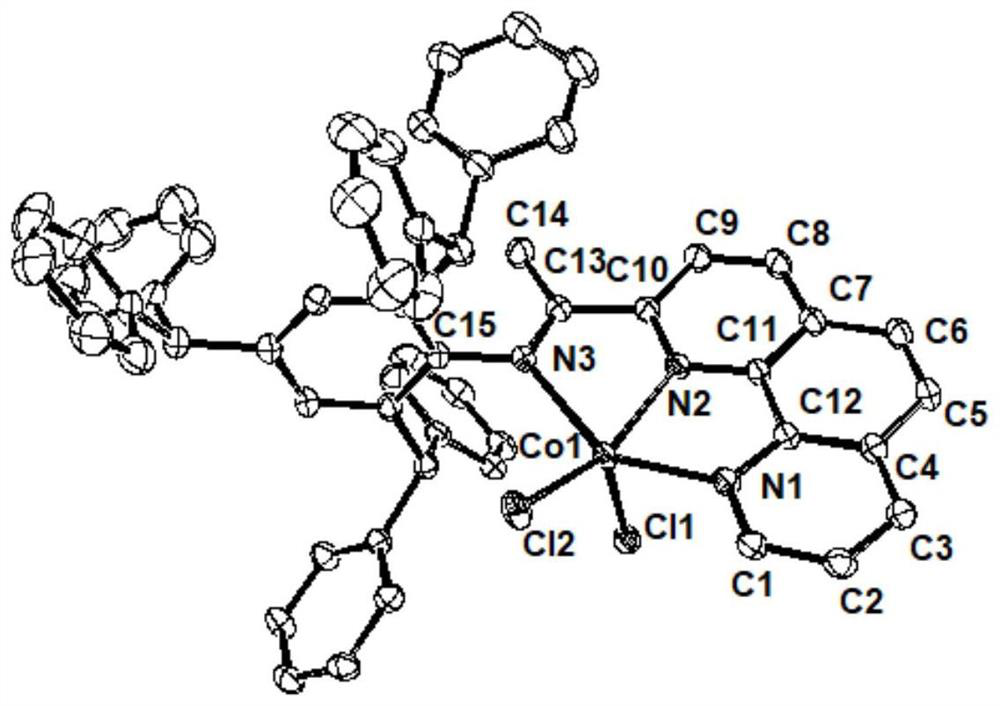
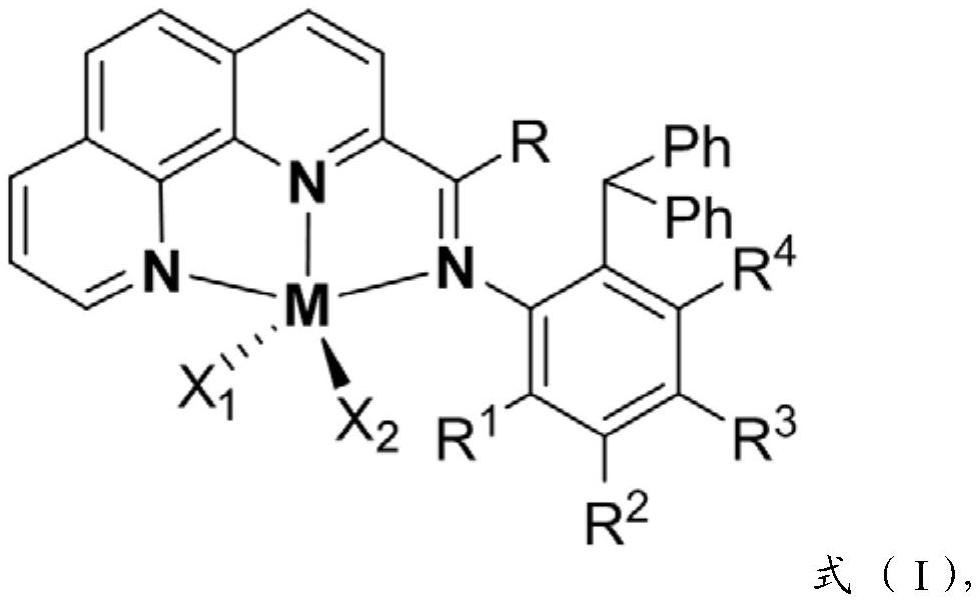
2-imine-1,10-phenanthroline metal complex catalyst containing aromatic hydrocarbon substituent as well as preparation method and application of 2-imine-1,10-phenanthroline metal complex catalyst
A technology of complexes and substituents, applied in the field of transition metal complexes of 2-imine-1,10-phenanthroline, can solve the problems of easy deactivation, reduced activity, poor thermal stability of complexes, etc. Low, stable performance, short cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0113] The preparation method of the 2-imine-1,10-phenanthroline transition metal complex containing an aromatic substituent comprises the following steps:
[0114] Step 1. Dissolving 2-imine-1,10-phenanthroline containing an aromatic substituent and a compound containing a transition metal in solvent I, stirring and reacting to obtain a reaction solution.
[0115] The structural formula of the 2-imine-1,10-phenanthroline containing an aromatic substituent is formula (II).
[0116] The transition metal-containing compound is selected from iron-containing compounds, cobalt-containing compounds, chromium-containing compounds or nickel-containing compounds, preferably selected from cobalt-containing compounds or iron-containing compounds, more preferably cobalt-containing halides or iron-containing halides.
[0117] Preferably,
[0118] The cobalt-containing halide is selected from cobalt bromide or cobalt chloride, preferably cobalt chloride,
[0119] The iron-containing halid...
Embodiment 1
[0164] Weigh 0.79g of 2-acetyl-1,10-phenanthroline and 1.32g of 2,6-bis(benzhydryl)-4-methylaniline into the reaction flask and dissolve them in 40mL of toluene solvent A mixed solution was formed, and then 0.2 g of p-toluenesulfonic acid was weighed and added into the reaction flask, and dissolved uniformly to form a reaction mixture. The reaction mixture was heated to 110°C, refluxed, and reacted for 16h. After cooling to room temperature, the solvent was distilled off under reduced pressure. The resulting crude product was dissolved in 5 mL of dichloromethane, and subjected to column chromatography through a basic alumina column, eluting with a mixed solvent of petroleum ether and ethyl acetate (25:1 by volume) as an eluent, and collected For the second component, the solvent was distilled off under reduced pressure to obtain 0.56 g of yellow powder, the product was L1, and the yield was 28.8%.
Embodiment 2
[0166] Weigh 0.79g of 2-acetyl-1,10-phenanthroline and 1.36g of 2,6-bis(benzhydryl)-4-ethylaniline into the reaction flask and dissolve them in 40mL of toluene solvent A mixed solution was formed, and then 0.2 g of p-toluenesulfonic acid was weighed and added into the reaction flask, and dissolved uniformly to form a reaction mixture. The reaction mixture was heated to 110°C, refluxed, and reacted for 16h. After cooling to room temperature, the solvent was distilled off under reduced pressure. The obtained crude product was dissolved in 5-10 mL of dichloromethane, and subjected to column chromatography through a basic alumina column, and a mixed solvent of petroleum ether and ethyl acetate (25:1 by volume) was used as eluent for eluting , collected the second component, and distilled off the solvent under reduced pressure to obtain 0.60 g of yellow powder, the product was L2, and the yield was 30.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com