Preparation method of catalyst for photocatalytic decomposition of pure water
A catalyst, photocatalysis technology, applied in the field of photochemical modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
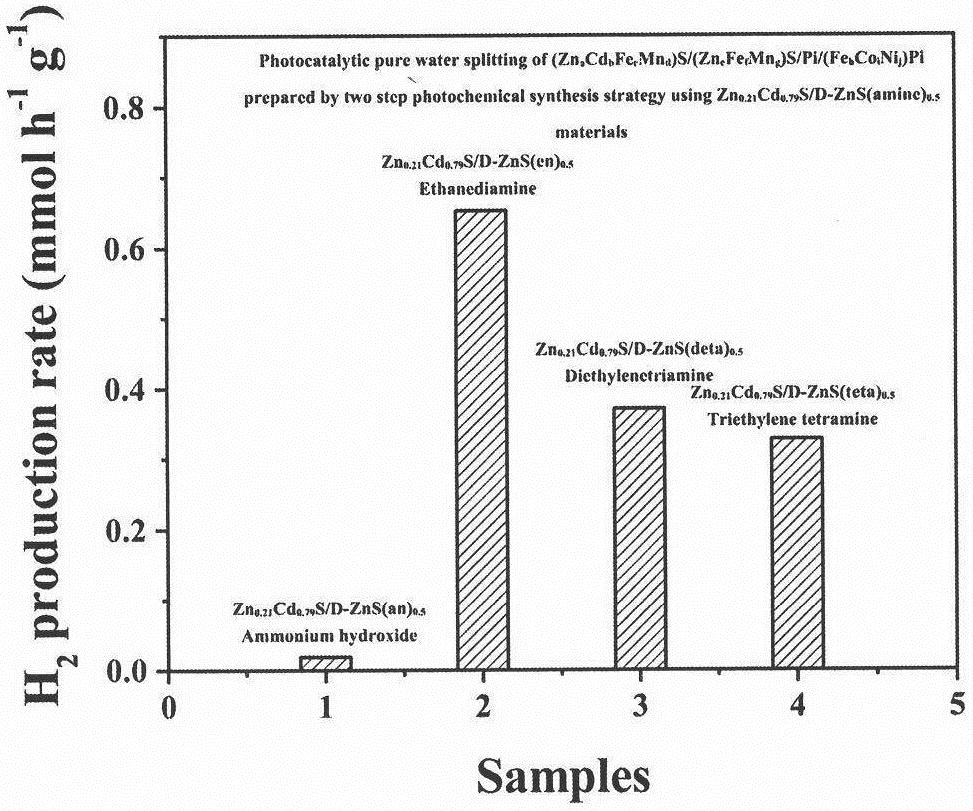
[0042] Example 1. Zn 0.21 Cd 0.79 S / D-ZnS(en) 0.5 Synthesis of heterojunction materials
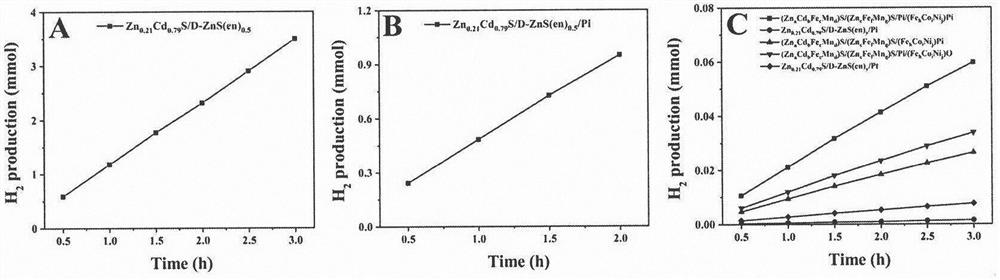
[0043] Mix 0.5mmol zinc acetate, 0.5mmol cadmium acetate and 2mmol L-cysteine in a mixed solvent of ethylenediamine: ultrapure water = 31.5ml: 1.5ml, and after ultrasonic stirring for 30min, the uniformly stirred suspension Transfer to the reactor and react at 180°C for 24h. Then centrifuge, wash the precipitated matter, and dry it in an oven at 60°C for 12 hours to recover a yellow sample, marked as Zn 0.21 Cd 0.79 S / D-ZnS(en) 0.5 .
Embodiment 2
[0044] Example 2. Zn 0.21 Cd 0.79 S / D-ZnS(en) 0.5 The first step of photochemical synthesis modification of heterojunction materials
[0045] Weigh 160mg Zn 0.21 Cd 0.79 S / D-ZnS(en) 0.5 Sample, dispersed in 80ml, 0.28125M NaH 2 PO 2 In aqueous solution, under the protection of argon, sonicate for 30min. Then the suspension was transferred to a photocatalytic reactor, and after the container was sealed, the whole reaction system was vacuumed for 15 min with a vacuum pump. Utilize a visible light source (300W xenon lamp equipped with a 420nm front cut-off filter) to irradiate the reactor for 3h, keep room temperature, and keep stirring. Molecular sieve column) for quantitative analysis. After the reaction is over, open the reactor, remove the supernatant, centrifuge to recover the precipitate, dry it in an oven at 60°C for 12 hours and recover a yellow sample, marked as Zn 0.21 Cd 0.79 S / D-ZnS(en) 0.5 / Pi.
Embodiment 3
[0046] Example 3. Zn 0.21 Cd 0.79 S / D-ZnS(en) 0.5 / The second step photochemical synthesis modification of Pi materials
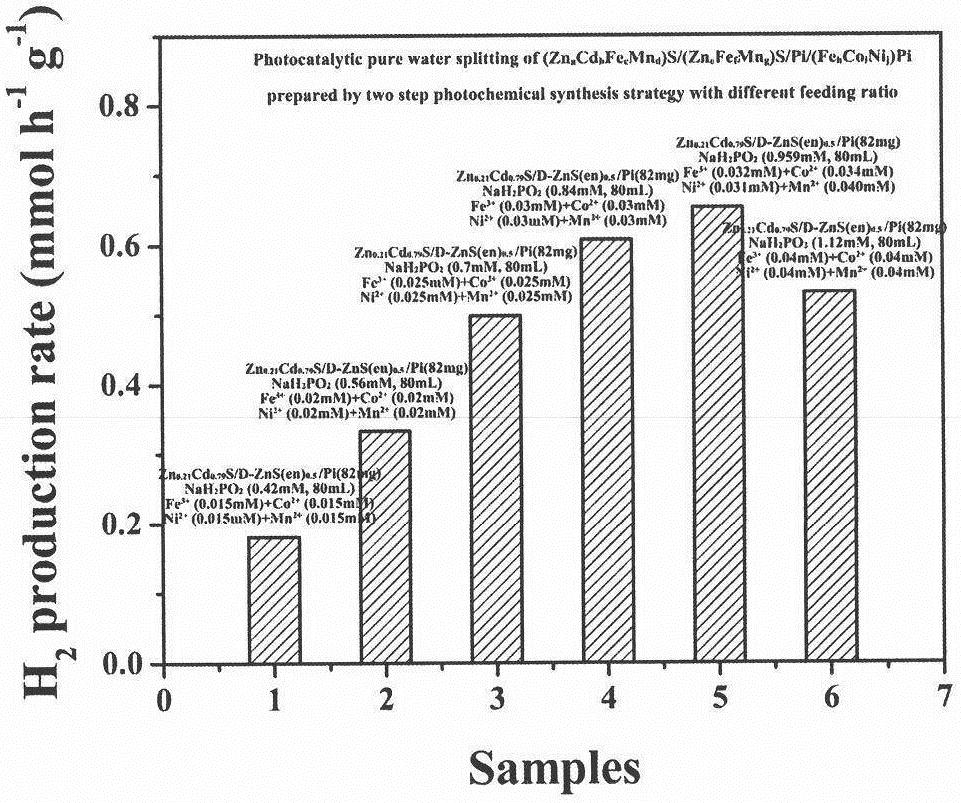
[0047] Weigh 82mg Zn 0.21 Cd 0.79 S / D-ZnS(en) 0.5 / Pi sample, dispersed into 80ml of metal salts and NaH with different concentrations 2 PO 2 In the aqueous solution, the specific feeding combination is as follows:
[0048] ①Fe(NO 3 ) 3 9H 2 O(0.015mM), Co(NO 3 ) 2 ·6H 2 O(0.015mM), NiSO 4 ·6H 2 O(0.015mM), MnSO 4 ·H 2 O(0.015mM) and NaH 2 PO 2 (0.42mM);
[0049] ②Fe(NO 3 ) 3 9H 2 O(0.020mM), Co(NO 3 ) 2 ·6H 2 O (0.020mM), NiSO 4 ·6H 2 O(0.020mM), MnSO 4 ·H 2 O(0.020mM) and NaH 2 PO 2 (0.56mM);
[0050] ③Fe(NO 3 ) 3 9H 2 O(0.025mM), Co(NO 3 ) 2 ·6H 2 O(0.025mM), NiSO 4 ·6H 2 O(0.025mM), MnSO 4 ·H 2 O(0.025mM) and NaH 2 PO 2 (0.70mM);
[0051] ④Fe(NO 3 ) 3 9H 2 O(0.030mM), Co(NO 3 ) 2 ·6H 2 O (0.030mM), NiSO 4 ·6H 2 O(0.030mM), MnSO 4 ·H 2 O(0.030mM) and NaH 2 PO 2 (0.84mM);
[0052] ⑤Fe(NO 3 ) 3 9H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lattice spacing | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com