Oxindole compound as well as preparation method and application thereof
A technology of oxindole and compounds, applied in organic chemistry, drug combination, antineoplastic drugs, etc., to achieve the effects of strong compatibility, economical reaction steps, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
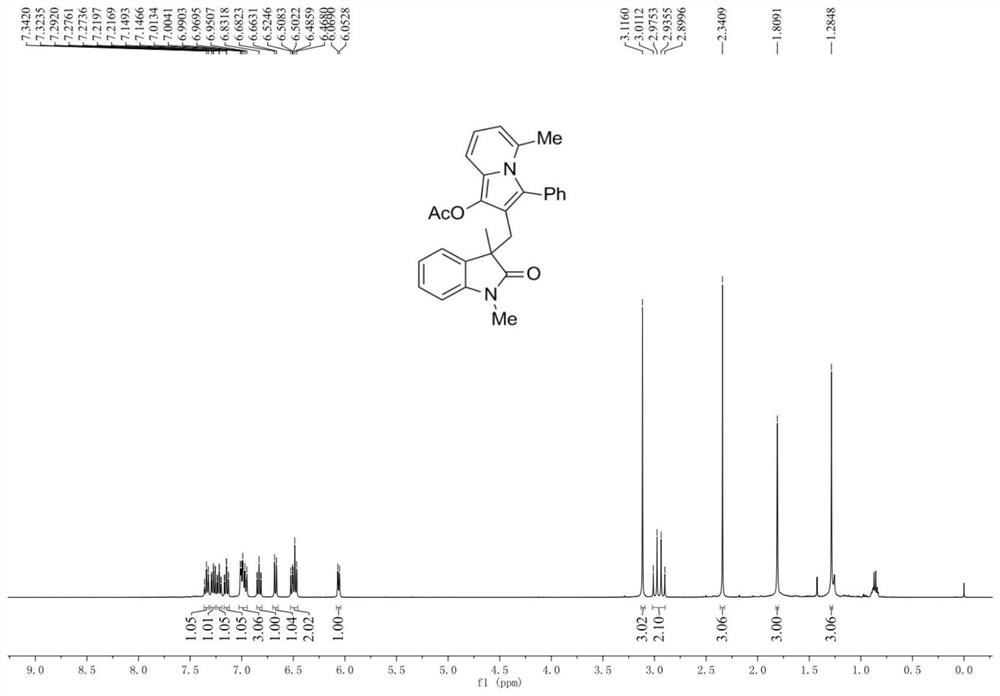
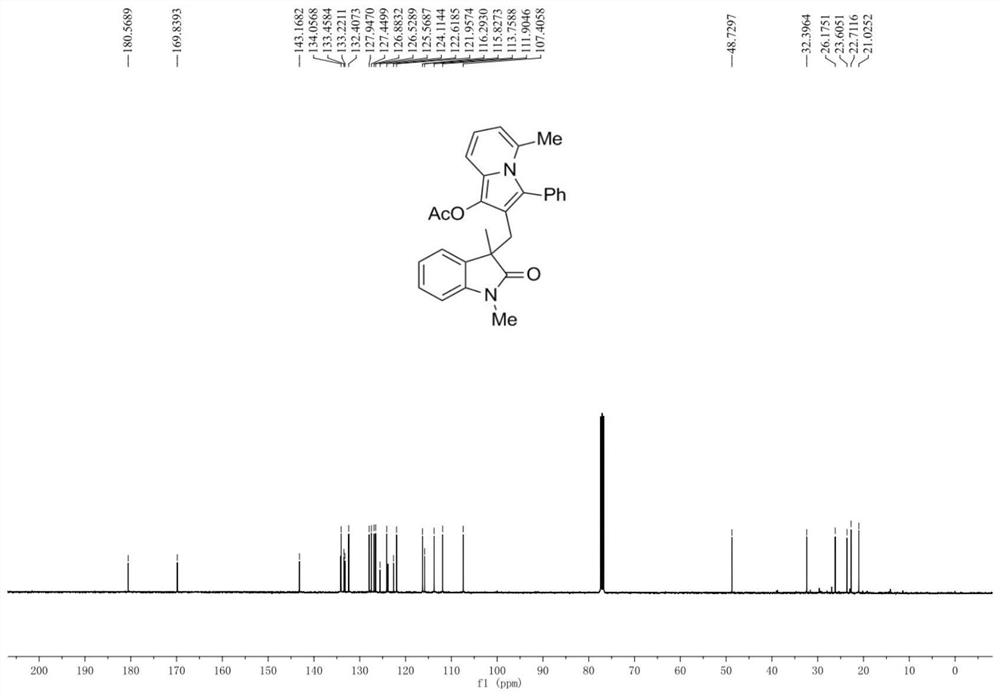
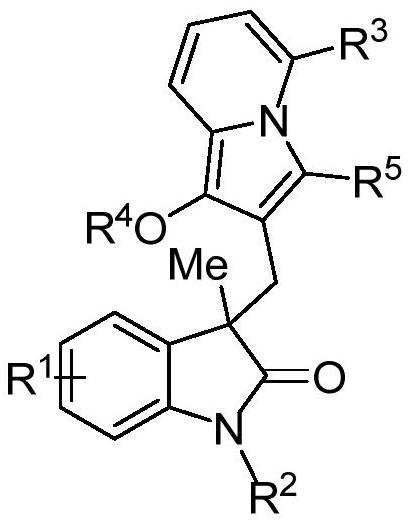
[0041] An oxidindole compound, having the structural formula shown in A1:
[0042]
[0043] The preparation method of above-mentioned oxindole compounds is as follows:
[0044] Add 0.2 mmol N-(2-iodophenyl)-N-methylmethacrylamide, 0.26 mmol 1-(6-methylpyridin-2-yl)-3-phenylacetylene to the reaction tube Propyl ester, 0.01 mmol tetrakis(triphenylphosphine) palladium, 0.4 mmol potassium carbonate, 2 ml N,N-dimethylformamide, reacted at 80°C under nitrogen protection for 6 hours, stopped the reaction and cooled to room temperature, added With 5 milliliters of water, the reaction solution was extracted three times with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain a crude product, which was subjected to column chromatography (eluent had a volume ratio of 7: 1 mixed solvent of petroleum ether and ethyl acetate) separation and purification to obtain the target produc...
Embodiment 2
[0047] A kind of oxindole compound, its structural formula is consistent with embodiment 1.
[0048] The difference between the preparation method of the oxindole compound and Example 1 is that toluene is used as the organic solvent in this example, and the yield is 59%.
Embodiment 3
[0050] An oxidindole compound whose structural formula is consistent with that of Example 1.
[0051] The difference between the preparation method of the oxindole compound and Example 1 is that acetonitrile is used as the organic solvent in this example, and the yield is 42%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com