Separation method of human intervertebral disc PROCR < + > nucleus pulposus progenitor cells
A separation method and intervertebral disc technology, applied in the field of isolation of human intervertebral disc PROCR+ nucleus pulposus progenitor cells, can solve the problems of simple cell components in the nucleus pulposus, complex induction technology, and remote clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1 solution preparation
[0083] 1. 1× red blood cell lysate:
[0084] with ddH 2 Dilute 10× red blood cell lysate with O and store at 4°C
[0085] 2. Configure 0.2% (w / v) of Pronase:
[0086] 0.1 g was added to 50 mL of distilled water, and the solution was filtered with a 0.22-μm membrane filter, and stored at 4°C.
[0087] 3. Prepare 0.1% (w / v) collagenase II:
[0088] Weigh 0.05g of collagenase II, add 50mL of serum-free DMEM / F12, filter the solution with a 0.22-μm membrane filter, and prepare it for immediate use.
[0089] 4. Prepare 2% penicillin and streptomycin in PBS:
[0090] Take 1mL penicillin and streptomycin and 49mL PBS with a pipette gun, mix well, and store at 4°C.
[0091] 5. Prepare PBS containing 3% BSA:
[0092] Pipette 1.5mL BSA into 48.5mL PBS, mix well, and store at 4°C.
[0093] Embodiment 2 intervertebral disc cell digestion
[0094] 1. Sample acquisition:
[0095] Avoid electrocoagulation of disc tissue during disc surgery ...
Embodiment 3
[0110] Example 3 Cell Quality Inspection
[0111] 1. AO / PI staining:
[0112] Add 1 mL of 2% penicillin and streptomycin solution in PBS to resuspend the cells, pipette 10 ul of the cell suspension and 10 ul of the AO / PI solution to mix at a ratio of 1:1, and stain for 2 minutes.
[0113] 2. Cell count:
[0114] Aspirate 20ul of the stained cell suspension and count with a Counter Star counter.
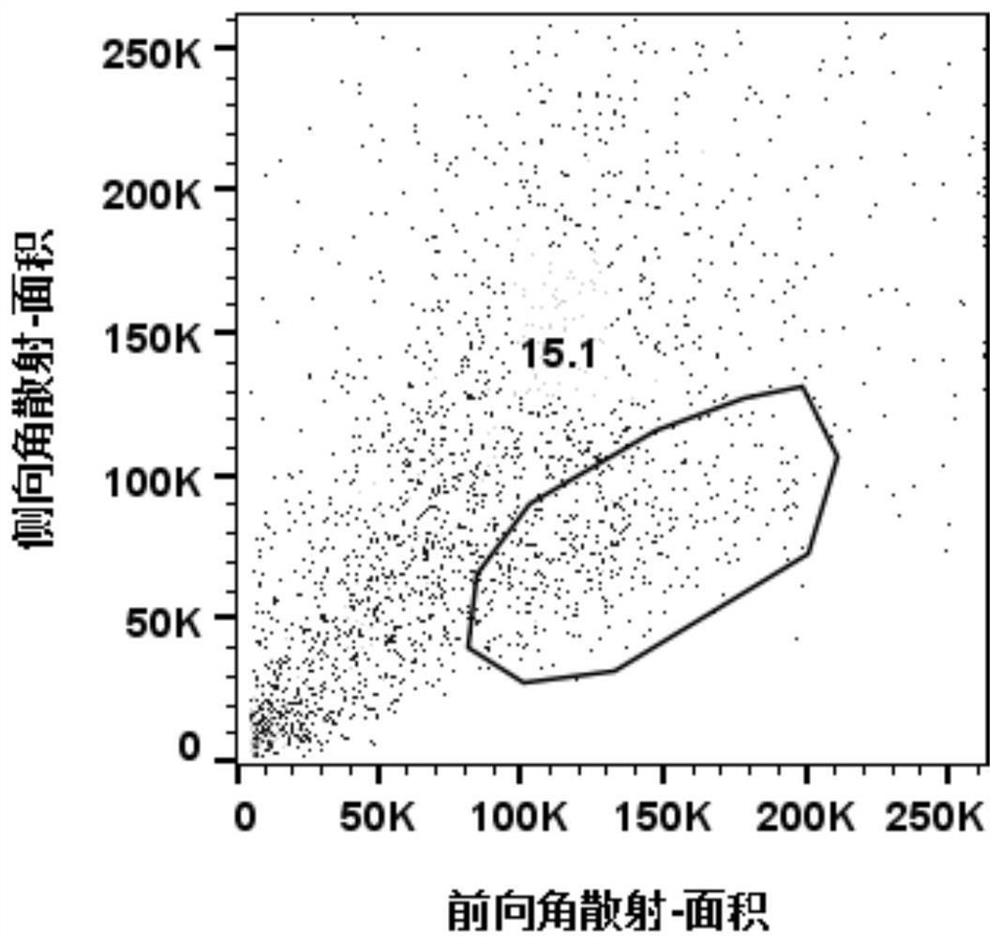
[0115] The result is as figure 1 As shown, the measured cell viability was 81.35%, and the number of cells was 1.01×10 6 , the light microscope photos show that the background is not clear, the color of the cell pellet is not uniform, and there are many impurities. Magnetic bead sorting is required to remove impurities.
Embodiment 4
[0116] Example 4 Magnetic bead sorting to remove impurities
[0117] 1. Preparation of reagents
[0118]Prepare 1×PBS containing 0.04% BSA, store at 4°C
[0119] ddH 2 O Prepare 1×Binding Buffer
[0120] 2. Remove tissue impurities
[0121] 1) Separation column selection:
[0122] Miltenyi Biotec MS columns require the total number of cells to be 2×10 8 Within, and the number of dead cells is 1×10 7 Within; Miltenyi Biotec LS columns require the total number of cells to be within 2×10 9 Within, and the dead cells are within 1×10 8 within.
[0123] 2) Take no more than 1×10 7 dead cells and 2×10 8 Within the total number of cells, centrifuge at 4°C at 300g / 5min to collect the cells and discard the supernatant.
[0124] 3) Resuspend the cells with 100ul Dead Cell Removal Micro Beads, mix gently and incubate at room temperature for 15min.
[0125] 4) Adsorb the MACS Separator to the MACS MultiStand bracket, select a suitable type of column to adsorb on the separator, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com