Pharmaceutical composition for treating Crohn's disease and preparation method thereof
A technology for Crohn's disease and a composition is applied in the field of pharmaceutical compositions for treating Crohn's disease and their preparation, which can solve the problems of poor tolerance and easy rejection, achieve better immunosuppressive function, and promote CD healing. , the effect of reducing complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] An injection for treating moderate to severe Crohn's disease, comprising umbilical cord mesenchymal stem cells and physiological saline containing 5% (volume fraction) albumin.
[0040]The method for extracting umbilical cord mesenchymal stem cells comprises the following steps: ligating both ends of fresh umbilical cord tissue, soaking in a sterile bottle containing physiological saline, and transporting it to a laboratory at 2-8°C; Open the bottle, discard the preservation solution in the bottle, add physiological saline (just immerse the umbilical cord) into the bottle, shake and wash. After discarding the saline, pour the umbilical cord into a sterile 100mm Petri dish and spray the surface of the umbilical cord with 75% ethanol solution. After cleaning the umbilical cord after alcohol spraying with normal saline for 2-3 times, add normal saline to the petri dish, take sterile scissors and divide the umbilical cord into 3-4cm segments, and wash away the blood; put th...
Embodiment 2
[0043] Animal experiment:
[0044] 1. Experimental method:
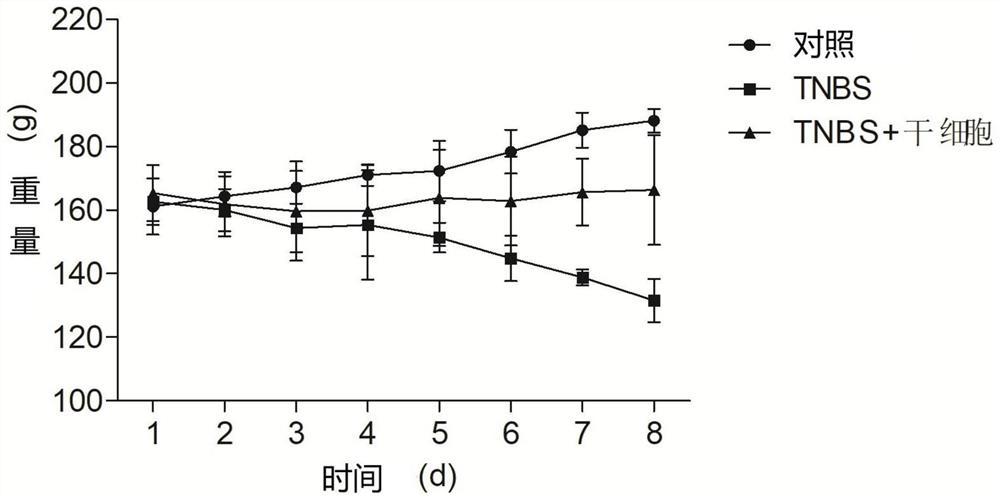
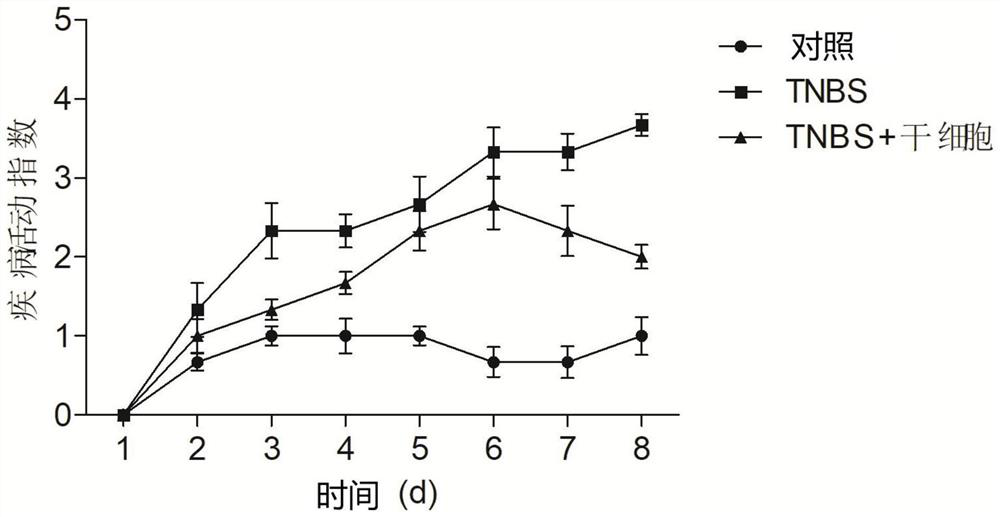
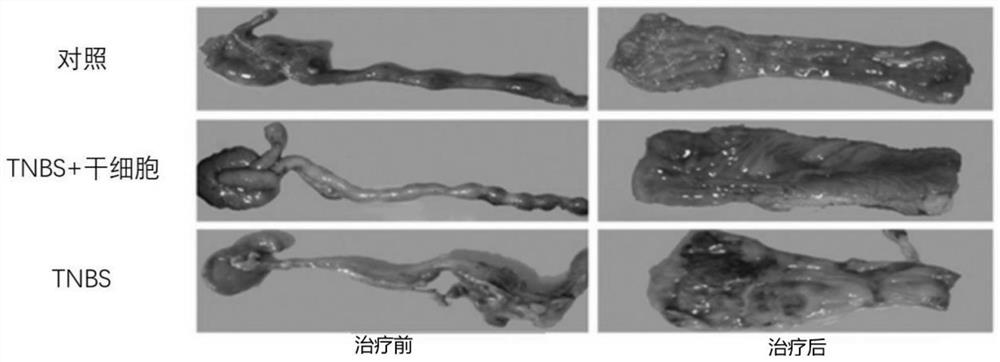
[0045] Thirty specific pathogen-free (SPF) female Sprague-Dawley rats (6-8 weeks), purchased from Shanghai Slack Experimental Animal Co., Ltd., were adaptively fed for one week before the experiment, and the feeding conditions were: temperature 22±2°C, humidity 55±5%, normal diet. After fasting for 24 hours on D0 day, they were randomly divided into three groups (n=10 / group): control group, trinitrobenzenesulfonic acid (TNBS) group, and TNBS+stem cell group.
[0046] On D1 day, all rats were anesthetized by intraperitoneal injection of 3% pentobarbital sodium 1ml / kg body weight.
[0047] TNBS group: Mix 5% TNBS and absolute ethanol at a volume ratio of 1:1 to make a 50% ethanol solution containing 2.5 mg / ml TNBS, and enema at a distance of 8-10 cm from the anus according to the standard of 100 mg / kg body weight of the mixed solution After filling, pull out the hose and keep the rat upside down for about 30 seconds...
Embodiment 3
[0073] Different injection methods and doses are used to treat Crohn's disease.
[0074] Patients with Crohn's disease were screened and randomly divided into five groups of 4 people. Different drugs and administration methods were used to observe the effectiveness and safety of clinical remission of moderate to severe Crohn's disease.
[0075] The inclusion criteria of the subjects are as follows:
[0076] 1) At the time of randomization, subjects between the ages of 18-75 (including the boundary value), male or female,
[0077] 2) At the time of randomization, records showed that subjects with a diagnosis of ileal, colonic or ileocolic Crohn's disease had been present for at least 3 months.
[0078] 3) Currently suffering from Crohn's disease and the Crohn's disease activity index (CDAI) score ≥ 220 and ≤ 450 points.
[0079] 4) During the screening period, the endoscopic confirmatory examination of active disease confirms that the subject has evidence of active inflammati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com