Preparation method of human BMP2 (bone morphogenetic protein 2) and analogue thereof
A BMP2-T, DS-BMP2-T technology, applied in the field of genetic engineering, can solve the problems of complex process, low expression, low renaturation efficiency, etc., and achieve the effect of good process controllability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
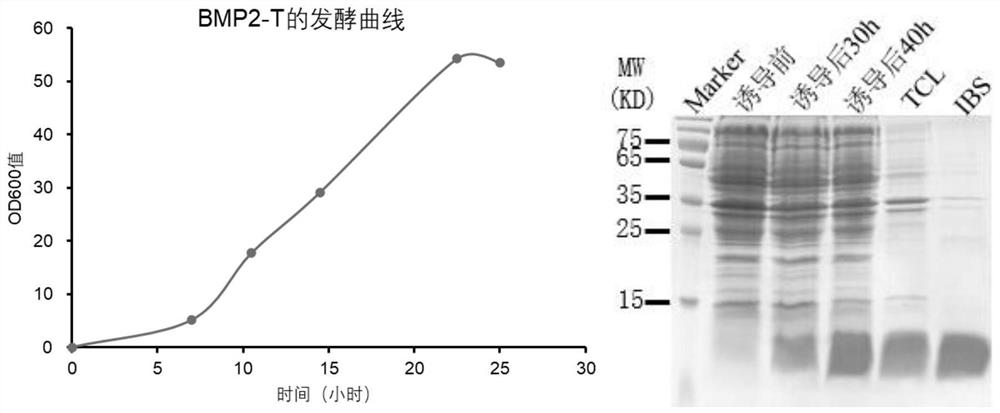
[0038] The preparation of embodiment 1 BMP2-T
[0039] (1) BMP2-T coding gene synthesis, vector construction and expression strain construction
[0040] 1.1 The 290-396th position of the gene encoding the BMP2 protein (283-396th position of unipro No.P12643), and introduce the R291K mutation modification, and replace the codon with the E. coli preference ( High-frequency use) codons to be suitable for expression in Escherichia coli, and add the initiation codon ATG before the optimized sequence, and finally obtain the optimized BMP2-T coding gene sequence as shown in the sequence table SEQ ID NO: 1 shown.
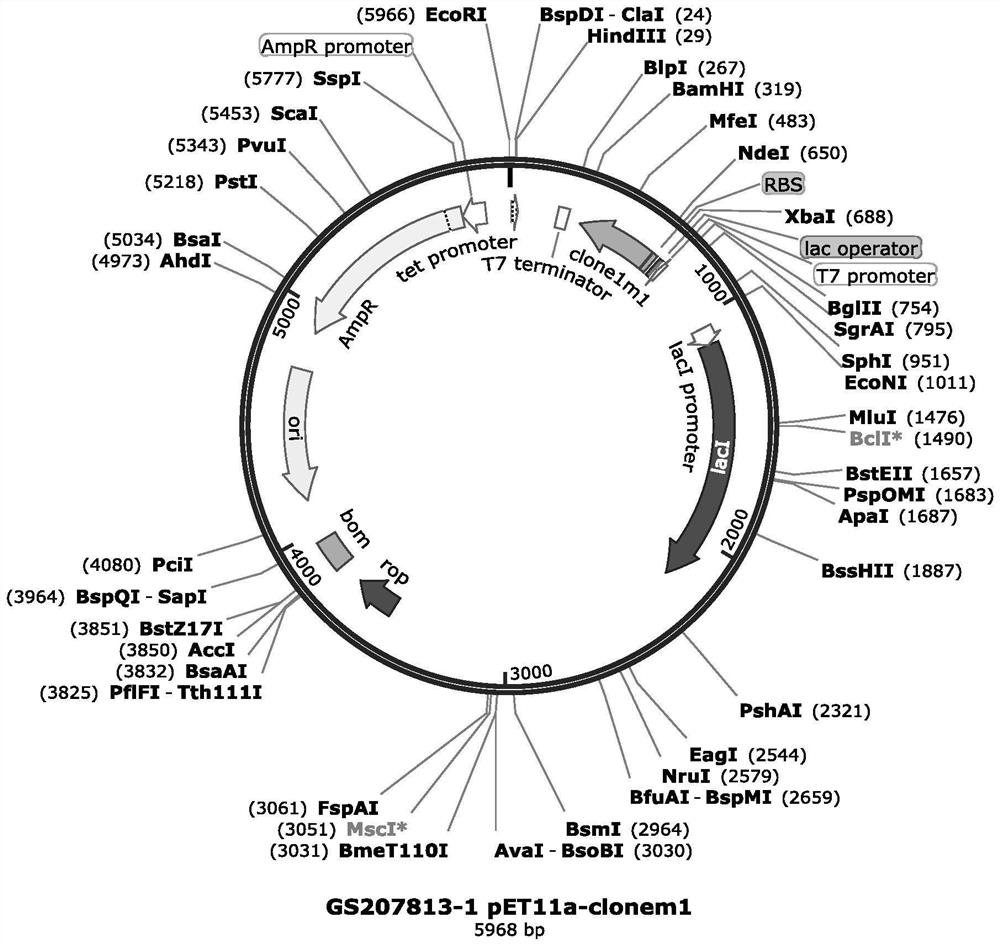
[0041] 1.2 Insert the double-stranded DNA molecule artificially synthesized by the coding strand nucleotides shown in SEQ ID NO: 1 in the sequence table through the principle of complementary base pairing into the NdeI / BamHI restriction site of the vector pET11a(+) to obtain a recombinant plasmid ( figure 1 ). In the recombinant plasmid, the inserted double-stranded DNA...
Embodiment 2
[0068] The preparation of embodiment 2 BMP2
[0069] 2.1 BMP2 encoding gene synthesis, vector construction and expression strain construction
[0070]The 283-396th position of the gene encoding the BMP2 protein (283-396th position of unipro No.P12643), without changing the amino acid sequence, replaced its codon with the preferred codon of Escherichia coli to be suitable for the large intestine Bacteria, and the start codon ATG was added before the optimized sequence to obtain the optimized BMP2 coding gene sequence as shown in the sequence table SEQ ID NO:3.
[0071] Insert the double-stranded DNA molecule artificially synthesized by the coding chain nucleotides shown in SEQ ID NO: 3 in the sequence table through the principle of complementary base pairing into the NcoI / XhoI restriction site of the vector pET-28a(+) to obtain the recombinant Plasmid ( Figure 8 ). In the recombinant plasmid, the inserted double-stranded DNA molecule is fused with part of the DNA of the pla...
Embodiment 3
[0082] Example 3 Preparation of DS-BMP2-T
[0083] 3.1 DS-BMP2-T coding gene synthesis, vector construction and expression strain construction
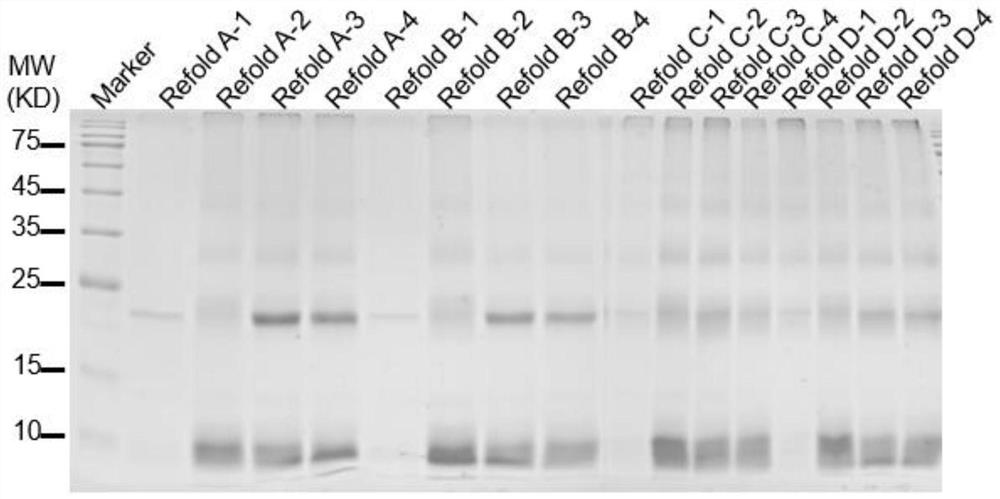
[0084] The 290-396th position of the gene encoding BMP2 protein (283-396th position of unipro No. P12643) is introduced into the R291K mutation modification. In order to improve the binding force of the BMP2 mutant to the collagen in the bone matrix, on the basis of the core protein sequence, a bone-binding polypeptide (sequence is DSSSSSSSSSSSSSSS) was introduced at the N-terminus of the protein, and the bone-binding polypeptide and the BMP2-T sequence The linking region of the protein was introduced into the linking sequence (sequence GSGS), in order to mention the expression of the protein, the high expression of the protein was finally obtained by fusing and expressing the form of TRX tag (thioredoxin protein) at the N-terminus. The final expressed protein sequence is shown in SEQ ID NO:6. Under the premise of not changing the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com