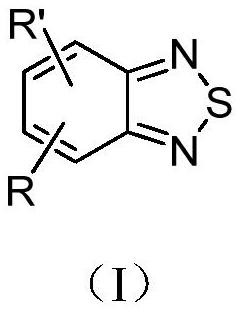
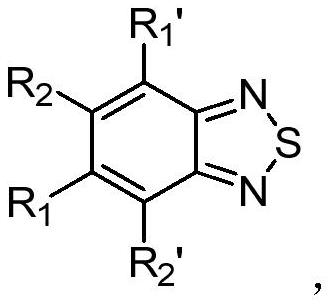
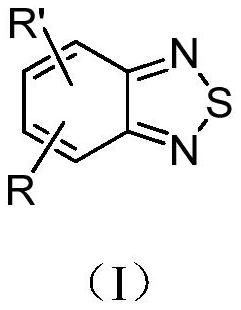
Application of benzothiadiazole compound in preparation of medicine for resisting SARS-COV-2 novel coronavirus
A technology of SARS-COV-2 and benzothiadiazoles, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of a benzothothiane compound
[0031]
[0032] Reagents and conditions: a) Dichloride, toluene; B) iron powder, ammonium chloride, ethanol, water; c) benzaldehyde, sodium borohydride, methanol.
[0033] Compound 15 (2 g, 13.07 mmol) of toluene (20 mL) was placed in three flasks (100 mL), and DMF (0.1 mL) and SOCL were sequentially added under ice bath. 2 (3.76 mL, 52.28 mmol), after the dropwise addition, the reaction solution was transferred into a 65 ° C oil bath, and the reaction was stirred for 3 h. After the monitoring reaction was complete, the filtrate was concentrated to give a purified compound 16 (2.2 g, yield 93.2%).
[0034] The compound 16 (2 g, 11.04 mmol) and NH 4 CL (2.34 g, 44.20 mmol) of ethanol and water (2: 1,3 ml) solution was placed in a 90 ° C oil bath for 30 min and then add iron powder (2.47 g, 44.20 mmol), and continued reflux and stirring reaction for 3h, monitoring After the reaction is complete, heat filtration, filter re...
Embodiment 2
[0059] Example 2 Benzothiane compound detection of SARS-COV-2 3ClPro protease activity:
[0060] Based on SARS-COV-2 3ClPro protein is the basic characteristics of proteolytic enzymes, and the screening system of SARS-COV-2 3ClPro protein activity is established by establishing fluorescence. SARS-COV-2 3Clpro protein specifically shear P1 bits of Gln (q) substrate, the activity detection can be used as a substrate to reflect the activity of the proteolytic enzyme by detecting the generation of fluorescent signals. SARS-COV-2 3ClPro protease activity Test uses fluorescent labeled polypeptide substrates (sequence: MCA-AVLQSGFRK (DNP) K), SARS-COV-2 3ClProase solution (diluted in the reaction liquid to 0.5 μm) and compound in reaction liquid (20 mm Tris, pH 7.3, 150 mM NaCl, 1 mM EDTA, 1% Glycerol, 0.01% Tween-20) was incubated for 10 minutes, and the substrate (40 μm, 50 μl of reaction liquid) was added, and the reaction was started after the reaction was used after Envision Multila...
Embodiment 3
[0062] Example 3 Benzothothiadiazole compounds for SARS-COV-2PLPRO protease activity inhibitory effect:
[0063] Based on SARS-COV-2PLPRO protein is the basic characteristics of proteolytic enzymes, and the screening system of SARS-COV-2PLPRO protein activity is established. SARS-COV-2 PLPRO protein can specifically identify and cut the bisglycine polypeptide, and the active detection can use a fluorescent polypeptide to react to the activity of its protein hydrolase by detecting the formation of fluorescent signals. SARS-COV-2PLPRO protease activity Test uses coumarin labeled polypeptide substrate (sequence: Z-RLRGG-AMC), SARS-COV-2PLPRO enzyme solution (diluted in the reaction liquid to 40 nm) and compound in reaction liquid (20 mm TRIS pH 8.0, 0.01% Tween 20, 0.5 mm DTT) The medium temperature was incubated for 10 minutes, and the substrate (50 μm, total volume of reaction liquid) was added, and the fluorescence intensity of the reaction liquid was detected using EnVision Multi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com