Domperidone sustained release preparation and sustained release carrier
A technology for domperidone and sustained-release preparations, applied in the field of medicine, can solve the problems of low bioavailability and the like, and achieve the effects of improving bioavailability, reducing toxicity, and good drug sustained-release performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation process of domperidone sustained-release preparation of the present invention comprises the following steps:
[0045] (1) Mix and ball-mill domperidone and sustained-release carrier first to obtain composite powder;
[0046] (2) The composite powder is then mixed with pharmaceutically acceptable excipients.
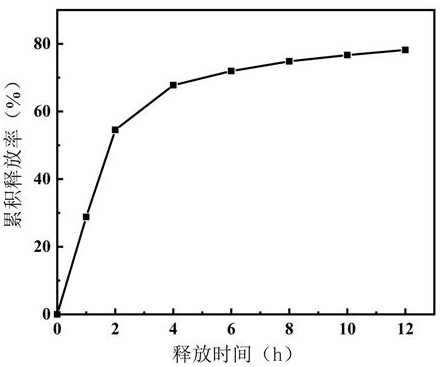
[0047] Compared with simultaneous mixing, domperidone is ball-milled and compounded with the slow-release carrier first, and then mixed with other excipients after compounding, which is beneficial to the mechanochemical effect of domperidone and the sodium attapulgite modified by vinylpyrrolidone as the slow-release carrier, so that Domperidone is firmly combined on the surface of the sustained-release carrier, reducing the occupation of other auxiliary materials on the surface of the sustained-release carrier, and providing stable release of the domperidone drug.
[0048] Take the tablet as a class, mix the compound powder and the pharmaceutically...
Embodiment 1
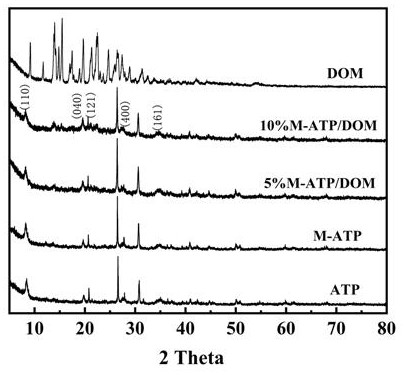
[0087] Step 1: Add 100g of attapulgite and 5g of sodium carbonate into 1.9 liters of deionized water together, at a stirring rate of 400-550 r min -1 Stir for 4-6 h under certain conditions to ion exchange sodium carbonate and attapulgite, then centrifuge, wash, dry, pulverize and sieve the mixed solution to obtain sodium attapulgite, marked as Na-ATP.
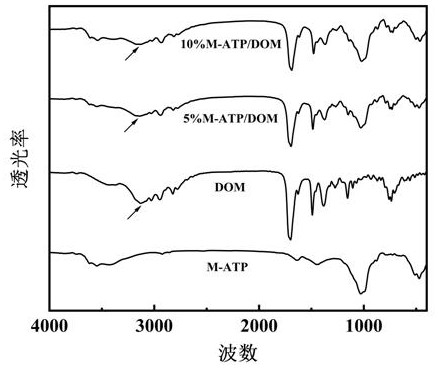
[0088] Step 2: Dissolve a certain amount of vinylpyrrolidone (NVP) in water, and add sodiumized attapulgite, wherein the concentration of sodiumized attapulgite is 10wt%, and the amount of vinylpyrrolidone is the mass of sodiumized attapulgite 10%, shaken at constant temperature for 3 hours, and then the mixed solution was filtered, washed with deionized water, dried, crushed, and sieved to obtain organic attapulgite, marked as M-ATP.
[0089] Step 3: Mix the organic attapulgite and domperidone in step 2 evenly at a mass ratio of 5:10, ball mill, dry and sieve to obtain a composite powder of organic attapulgite and domperidone...
Embodiment 2
[0092] Step 1: The preparation of sodium attapulgite is the same as in Example 1.
[0093] Step 2: Dissolving a certain amount of vinylpyrrolidone in water, adding sodiumized attapulgite, wherein the concentration of sodiumized attapulgite is 10wt%, and the amount of vinylpyrrolidone is 15% of the quality of sodiumized attapulgite, Shake at constant temperature for 3 hours, and then filter the mixed solution, wash with deionized water, dry, pulverize, and sieve to obtain organic attapulgite, which is marked as M-ATP.
[0094] Step 3: Mix the organic attapulgite and domperidone in step 2 evenly at a mass ratio of 10:10, ball mill, dry and sieve to obtain a composite powder of organic attapulgite and domperidone, labeled M-ATP / DOM. The particle size of the powder used for final tableting should not be less than 75 um.
[0095] Step 4: Through the direct powder compression method, the composite powder in step 3 is uniformly mixed with other excipients and then compressed into ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com