Modified boron nitride loaded nickel-based methane dry reforming catalyst as well as preparation method and application thereof
A technology for dry reforming of methane and boron nitride, applied in physical/chemical process catalysts, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of complex catalyst components, cumbersome preparation steps, environmental pollution, etc. Carbon deposition ability, simple preparation process, excellent anti-sintering effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
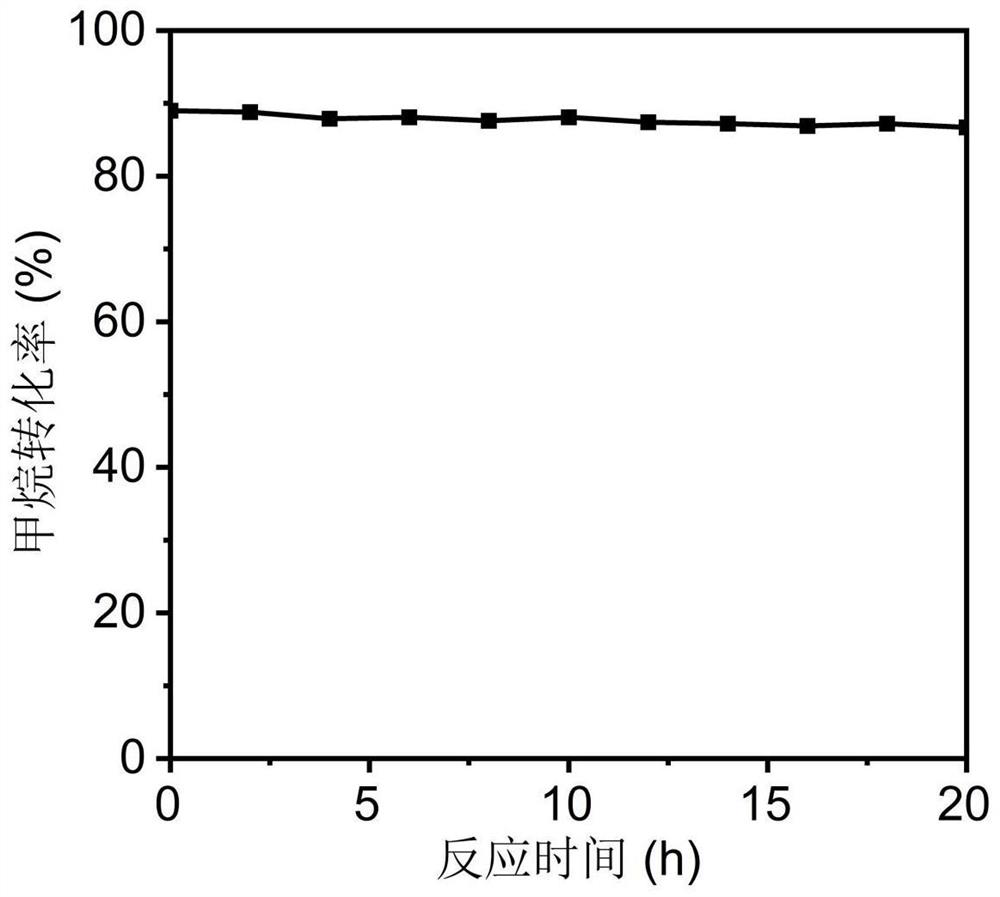
Image
Examples
Embodiment 1
[0029] In this embodiment, a method for preparing a modified boron nitride-supported nickel-based methane dry reforming catalyst has the following process steps:
[0030] a. Preparing the BN of the nickel-based methane dry reforming catalyst supported by modified boron nitride is peeled off by urea ball milling method, so that the surface is connected with amino functional groups, and the modified boron nitride product A is obtained;
[0031] b. Calculated according to the mass percentage, the nickel loading of the nickel-based methane dry reforming catalyst prepared by the modified boron nitride supported by the target is 5wt%, and the nickel nitrate and the boron nitride connected with the amino group are dispersed in the Stir vigorously in deionized water for 6 hours; then distill off the solvent and dry at 80°C for 10 hours; then in an air atmosphere, heat up at a rate of 2°C / min and heat up to 550°C for 6 hours to obtain Powder product B, as a precursor of nickel-methyl m...
Embodiment 2
[0039] In this embodiment, a method for preparing a modified boron nitride-supported nickel-based methane dry reforming catalyst has the following process steps:
[0040] a. The BN of the nickel-based methane dry reforming catalyst supported by modified boron nitride is peeled off in advance by alcohol ultrasonic method, so that the surface is connected with hydroxyl functional groups, and the modified boron nitride product A is obtained;
[0041] b. Calculated according to the mass percentage, the nickel loading of the nickel-based methane dry reforming catalyst loaded with modified boron nitride prepared by the target is 2wt%, and the nickel nitrate and the boron nitride connected with the hydroxyl group are dispersed in the Stir vigorously in deionized water for 4 hours; then distill off the solvent and dry at 60°C for 12 hours; then in an air atmosphere, heat up at a rate of 2°C / min and heat up to 600°C for 4 hours to obtain Powder product B, as a precursor of nickel-methy...
Embodiment 3
[0047] In this embodiment, a method for preparing a modified boron nitride-supported nickel-based methane dry reforming catalyst has the following process steps:
[0048] a. Preparing the BN of the nickel-based methane dry reforming catalyst supported by modified boron nitride is peeled off by urea ball milling method, so that the surface is connected with amino functional groups, and the modified boron nitride product A is obtained;
[0049] b. Calculated according to the mass percentage, the nickel loading of the nickel-based methane dry reforming catalyst prepared by the modified boron nitride supported by the target is 1wt%, and the nickel acetone acetate and the boron nitride with amino groups are weighed to disperse Stir vigorously in deionized water for 6 hours; then distill off the solvent and dry at 80°C for 12 hours; then in an air atmosphere, heat up at a rate of 1°C / min and heat up to 550°C for calcination for 5 hours. Obtain powder product B, as nickel base methan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com