Near-infrared nano photosensitizer as well as preparation method and application thereof
A near-infrared, photosensitizer technology, applied in the field of photosensitizers, can solve the problems of poor photostability, shallow treatment depth, etc., and achieve the effects of small polarizability, improved water solubility, and increased singlet oxygen yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
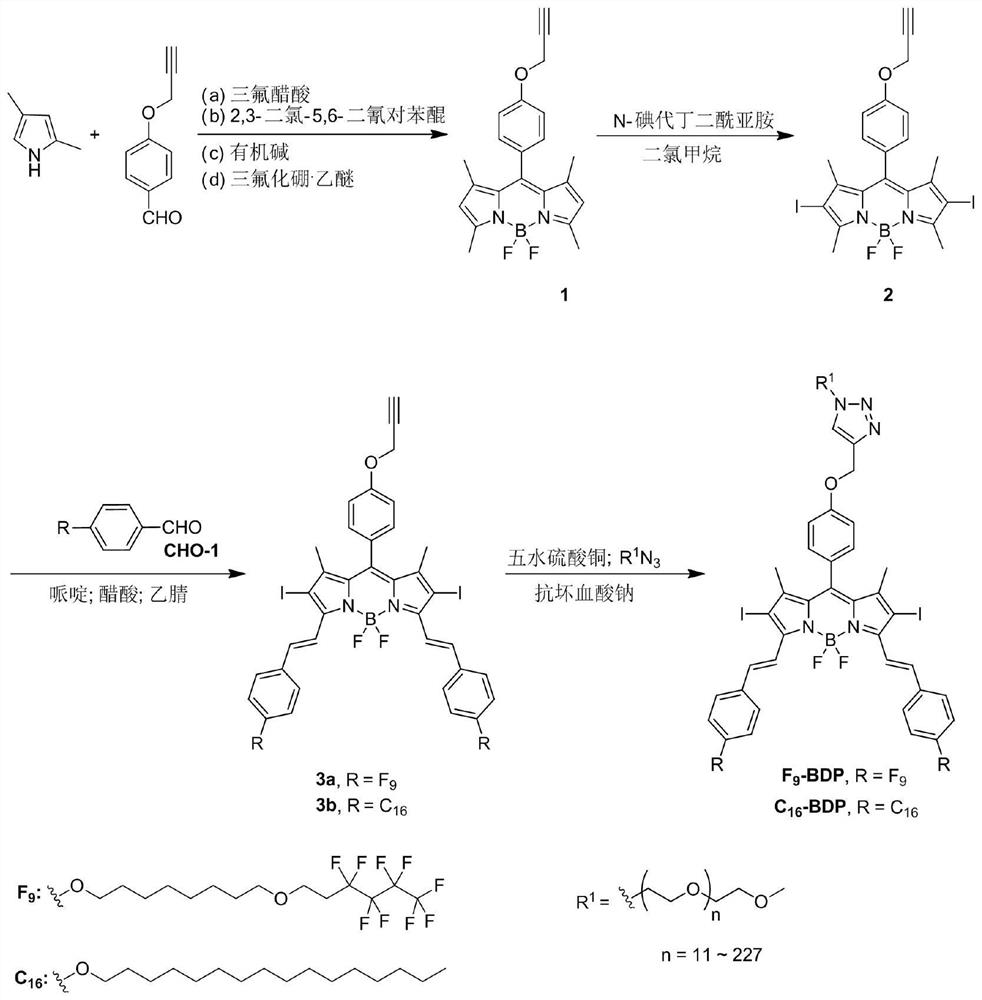
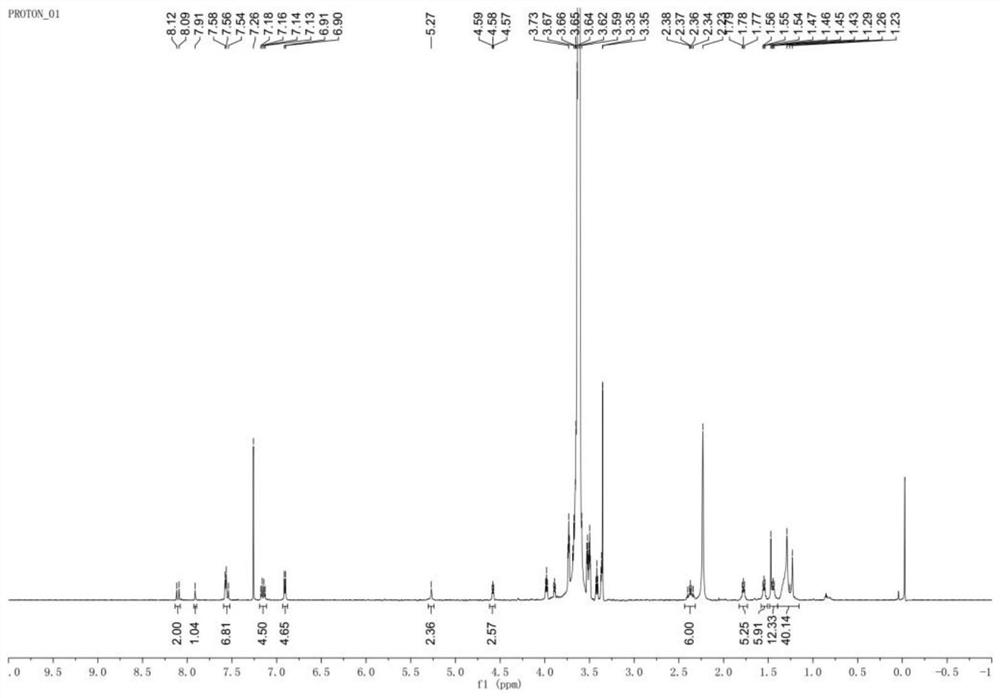
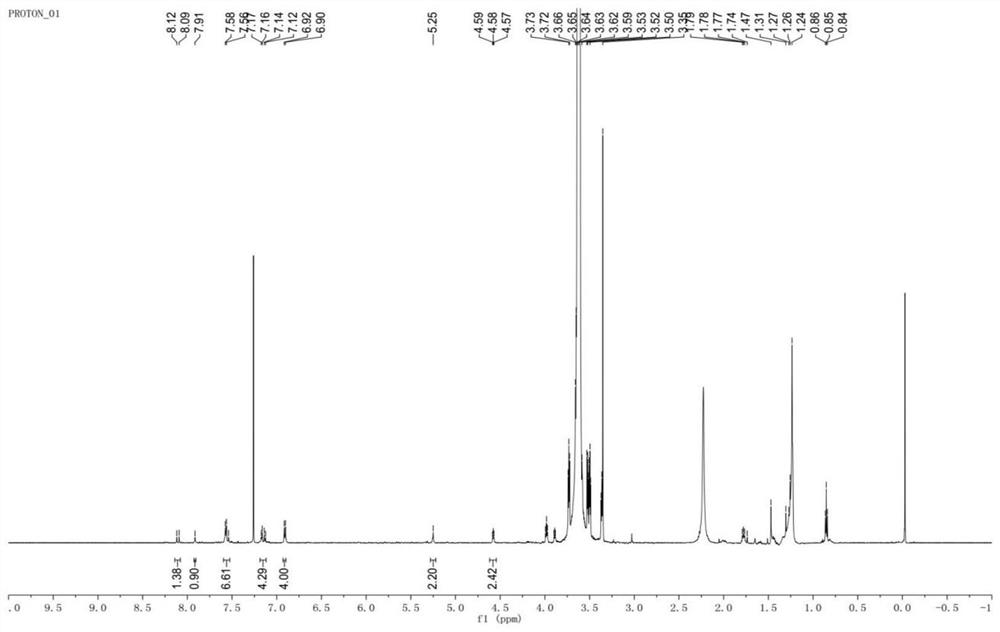
[0049] Such as figure 1 Shown, a kind of near-infrared nano photosensitizer and preparation method thereof, specifically comprise the following steps:
[0050] (1) Synthesis of Compound 1: Put alkyne-containing p-hydroxybenzaldehyde and 2,4-dimethylpyrrole in a molar ratio of 1:1 into the reaction vessel, and then add 100% of the weight of 2,4-dimethylpyrrole times THF as solvent and 6-10 drops of trifluoroacetic acid as catalyst. Stir at 25 °C for 24 h. Then, 2,3-dichloro-5,6-dicyano-p-benzoquinone with a molar ratio of 1:1.5 to 2,4-dimethylpyrrole was added to the above reaction solution, and stirred at room temperature for 24 hours. Finally, triethylamine 50 times the weight of 2,4-dimethylpyrrole was added, and boron trifluoride·ethyl ether 50 times the weight of 2,4-dimethylpyrrole was added dropwise in an ice-water bath to react overnight. After the reaction was finished, it was extracted with ethyl acetate and water, concentrated by a rotary evaporator, and the crude...
Embodiment 2
[0055] A near-infrared nano photosensitizer and a preparation method thereof, specifically comprising the following steps:
[0056] (1) Synthesis of compound 1: Add alkyne-containing p-hydroxybenzaldehyde and 2,4-dimethylpyrrole into the reaction vessel at a molar ratio of 1:3, and then add 50% of the weight of 2,4-dimethylpyrrole times THF as solvent and 3-5 drops of trifluoroacetic acid as catalyst. Stir at 40 °C for 24 h. Then, 2,3-dichloro-5,6-dicyano-p-benzoquinone with a molar ratio of 1:3 to 2,4-dimethylpyrrole was added to the above reaction liquid, and stirred at room temperature for 24 hours. Finally, triethylamine 100 times the weight of 2,4-dimethylpyrrole was added, and boron trifluoride·ethyl ether 100 times the weight of 2,4-dimethylpyrrole was added dropwise in an ice-water bath to react overnight. After the reaction was finished, it was extracted with ethyl acetate and water, concentrated by a rotary evaporator, and the crude product was obtained by flash co...
Embodiment 3
[0061] A near-infrared nano photosensitizer and a preparation method thereof, specifically comprising the following steps:
[0062] (1) Synthesis of Compound 1: Put alkyne-containing p-hydroxybenzaldehyde and 2,4-dimethylpyrrole in a molar ratio of 1:5 into the reaction vessel, and then add 30% of the weight of 2,4-dimethylpyrrole times dichloromethane as solvent and 6-10 drops of trifluoroacetic acid as catalyst. Stir at 60 °C for 48 h. Then, 2,3-dichloro-5,6-dicyano-p-benzoquinone with a molar ratio of 1:2 to 2,4-dimethylpyrrole was added to the above reaction liquid, and stirred at room temperature for 24 hours. Finally, triethylamine 50 times the weight of 2,4-dimethylpyrrole was added, and boron trifluoride·ethyl ether 50 times the weight of 2,4-dimethylpyrrole was added dropwise in an ice-water bath to react overnight. After the reaction was finished, it was extracted with ethyl acetate and water, concentrated by a rotary evaporator, and the crude product was obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com