Method for synthesizing cefuroxime sodium based on continuous flow reaction technology
A technology of cefuroxime sodium and cefuroxime acid, which is applied in the field of synthesizing cefuroxime sodium based on continuous flow reaction technology, can solve the problems of producing a large amount of waste acid water, difficult operation, unfavorable environmental protection, etc., and achieves reduction in emissions and safety The effect of low sexual risk and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for synthesizing cefuroxime sodium based on continuous flow reaction technology, comprising the following steps:
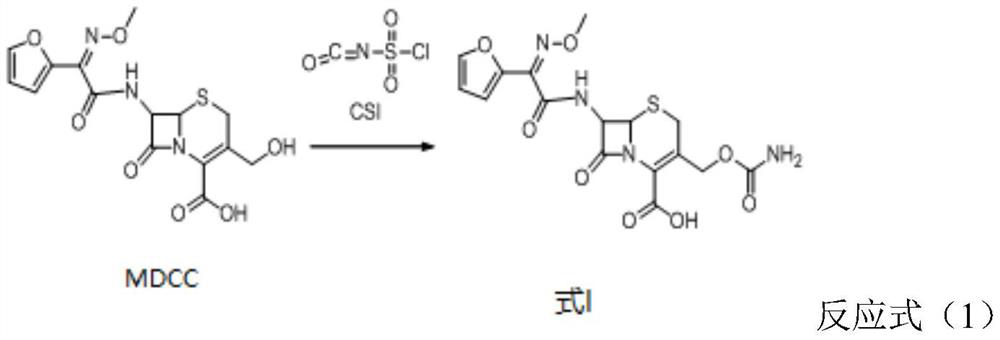
[0031] (1) Dissolve decarbamyl cefuroxime (MDCC) in tetrahydrofuran (THF) with a mass fraction of 20%, and cool down to 10°C; cool down chlorosulfonyl isocyanate (CSI) to 10°C. At the same time, the temperature of the continuous flow reactor was lowered to 10°C in advance; the continuous flow reactor was washed with tetrahydrofuran to ensure that the reactor pipeline was free of water.
[0032] (2) The tetrahydrofuran solution of decarbamyl cefuroxime (MDCC) and chlorosulfonyl isocyanate (CSI) are passed into the liquid inlet pipe respectively, and enter the continuous flow reactor simultaneously after mixing at the mixer place, and the controlled reaction temperature is 10 ℃, the reaction time is 45s. After calculation, the molar ratio of decarbamyl cefuroxime (MDCC) to chlorosulfonyl isocyanate (CSI) was 1:1.15.
[0033] (3) After quenching the...
Embodiment 2
[0037] A method for synthesizing cefuroxime sodium based on continuous flow reaction technology, comprising the following steps:
[0038] 1) Dissolve decarbamyl cefuroxime (MDCC) in tetrahydrofuran (THF) with a mass fraction of 20%, and cool down to 5°C; cool down chlorosulfonyl isocyanate (CSI) to 5°C. At the same time, the temperature of the continuous flow reactor was lowered to 5 °C in advance; the continuous flow reactor was washed with tetrahydrofuran to ensure that the reactor pipeline was free of water.
[0039] (2) The tetrahydrofuran solution of decarbamoyl cefuroxime (MDCC) and chlorosulfonyl isocyanate (CSI) are passed into the liquid inlet pipe respectively, enter the continuous flow reactor simultaneously after mixing at the mixer place, and control the reaction temperature to be 5 ℃, the reaction time is 45s. After calculation, the molar ratio of decarbamyl cefuroxime (MDCC) to chlorosulfonyl isocyanate (CSI) was 1:1.15.
[0040] (3) After quenching the produc...
Embodiment 3
[0044] A method for synthesizing cefuroxime sodium based on continuous flow reaction technology, comprising the following steps:
[0045] 1) Dissolve decarbamyl cefuroxime (MDCC) in tetrahydrofuran (THF) with a mass fraction of 20%, and cool down to 0°C; cool chlorosulfonyl isocyanate (CSI) to 0°C. At the same time, the temperature of the continuous flow reactor was lowered to 0°C in advance; the continuous flow reactor was washed with tetrahydrofuran to ensure that the reactor pipeline was free of water.
[0046] (2) The tetrahydrofuran solution of decarbamoyl cefuroxime (MDCC) and chlorosulfonyl isocyanate (CSI) are passed into the liquid inlet pipe respectively, and after being mixed at the mixer, enter the continuous flow reactor simultaneously, and the reaction temperature is controlled to be 0 ℃, the reaction time is 45s. After calculation, the molar ratio of decarbamyl cefuroxime (MDCC) to chlorosulfonyl isocyanate (CSI) was 1:1.15.
[0047] (3) After quenching the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com